Question
Question: Which of the following reactions will not give either a major product? A) \(C{{H}_{3}}C{{H}_{2}}Cl...
Which of the following reactions will not give either a major product?
A) CH3CH2Cl+Ag2O(dry)→
B) (CH3)3CCl+CH3CH2O−Na+→
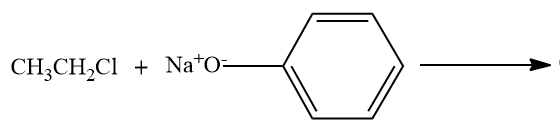
C)
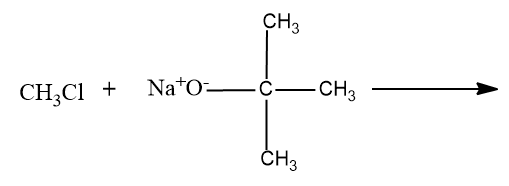
D)
Solution
The 3∘ carbocation, is the most reactive carbocation for the elimination reaction.
- The tertiary alkyl halides forms the tertiary carbocation during .
Complete step by step answer:
So in the question, it is asked which of the following options given will not give the major product as ether.
We should have to compare the reactions that happens for each options and we should know the product that forms to predict the major product.So lets see the different reaction-
- Option (A), CH3CH2Cl+Ag2O(dry)→
Here an alkyl halide,specifically saying a 1∘alkyl halide is reacting with the silver oxide ,here the product formed will be an ether and the ether is the major product formed here.
So the reaction is ,
2CH3CH2Cl+Ag2O(dry)→CH3CH2OCH3CH2(Majorproduct)+2AgCl
So this is not the option.
- Option (B), (CH3)3CCl+CH3CH2O−Na+→
In this case,here the reactants are tertiary Alkyl chain and sodium alkoxide.
Here the 3∘ carbon chain forms the 3∘ carbocation which cannot undergo the Williamson ether synthesis.
Since Williamson’s ether synthesis is a SN2 reaction .So 3∘carbocation are the least reactive carbocations towards the SN2 reaction.And it will undergo elimination reaction (E1 reaction) and the product formed will be alkene.
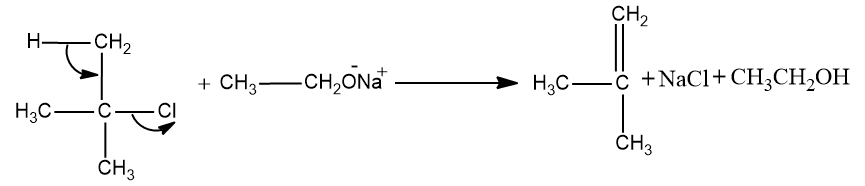
So this may be the correct option.
- Option C,
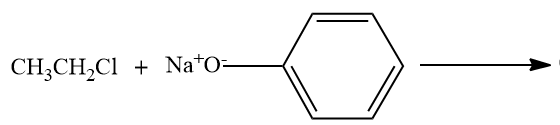
Here, the alkyl chloride ie ethyl chloride is reacting with the sodium phenoxide ion, and this chemical reaction forms the aryl-alkyl ether.Here the product formed is phenetole or called as Ethyl phenyl ether.
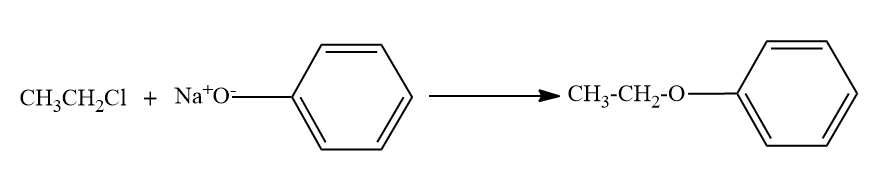
So here also ether is the major product formed.
- Option (D),
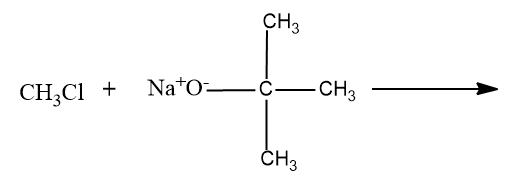
Here a primary carbocation will be formed during the chemical reaction and it undergoes the ether formation through Williamson’s ether synthesis.
So the product formed will be,
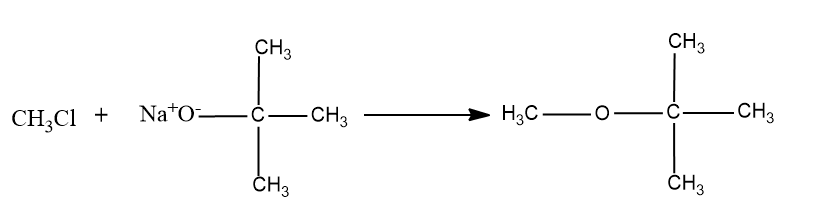
So this option is not the correct option.
From the above reactions we could conclude that the correct option is option (B), which gives alkene as the major product.
Note: We should know that for the ether formation through Williamson’s ether synthesis, the reactivity of the carbocation formed during the chemical reaction will be, 1∘>2∘>3∘
- And the Williamson’s ether synthesis is a SN2 reaction, hence the stability of the carbocations will be as given above.
- And the elimination reaction here ,the E1 reaction is similar to SN1 reaction and have a reactivity order of carbocations as, 3∘>2∘>1∘ and product formed here will be an alkene.
