Question
Question: Which of the following reactions will go faster if the concentration of nucleophiles is increased? ...
Which of the following reactions will go faster if the concentration of nucleophiles is increased?
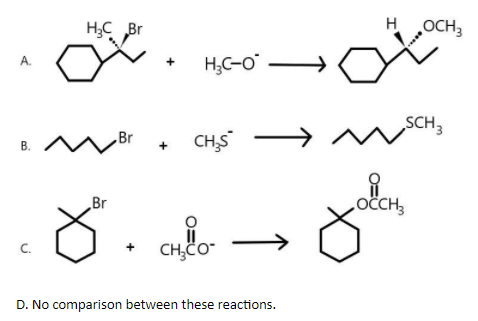
Solution
As we know that the given reactions are undergoing substitution nucleophilic unimolecular and bimolecular reactions. And we also know that the rate of a reaction is directly proportional to the concentration of a given nucleophile.
Complete step-by-step answer:
As we know that the first and second reactions are undergoing substitution nucleophile bimolecular reaction i.e. SN2 reaction where a single step bimolecular reaction is taking place in which the incoming nucleophile is attacking the carbon atom of the substrate in opposite direction to the outgoing nucleophile.
But in the third reaction, substitution nucleophile unimolecular reaction i.e. SN1 reaction is taking place where two step reaction happens. The first step involves the slow ionisation of substrate and the second step involves the reaction between the carbocation so formed and the nucleophile. As show below, bromine will first get removed and then methoxy group will be added as nucleophile.
We are also aware with the fact that rate of reaction in SN2 reaction is directly proportional to the concentration of a given nucleophile.Therefore, we can say that the first and second reactions will go faster if nucleophile concentration is increased.
Hence, the correct answers are (A) and (B).
Note: Always remember that the concentration is the sole factor on which the rate of a reaction is dependent in case of SN2 reactions only whereas in case of SN1 reaction, the rate of reaction depends upon the concentration of alkyl halide only and it is independent of the concentration of nucleophile. Further, the higher the stability of the carbocation, higher will be the ease of carbocation formation from alkyl halide and faster will be the rate of reaction.
