Question
Question: Which of the following reactions represent incorrect major products? (A) 
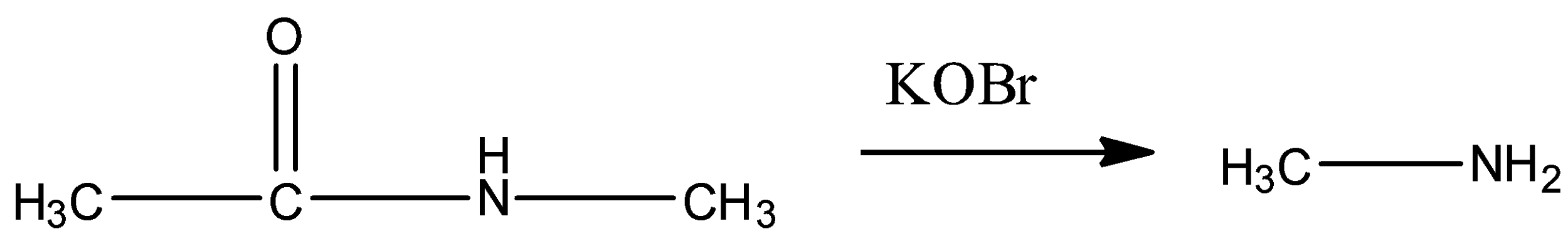
(B)
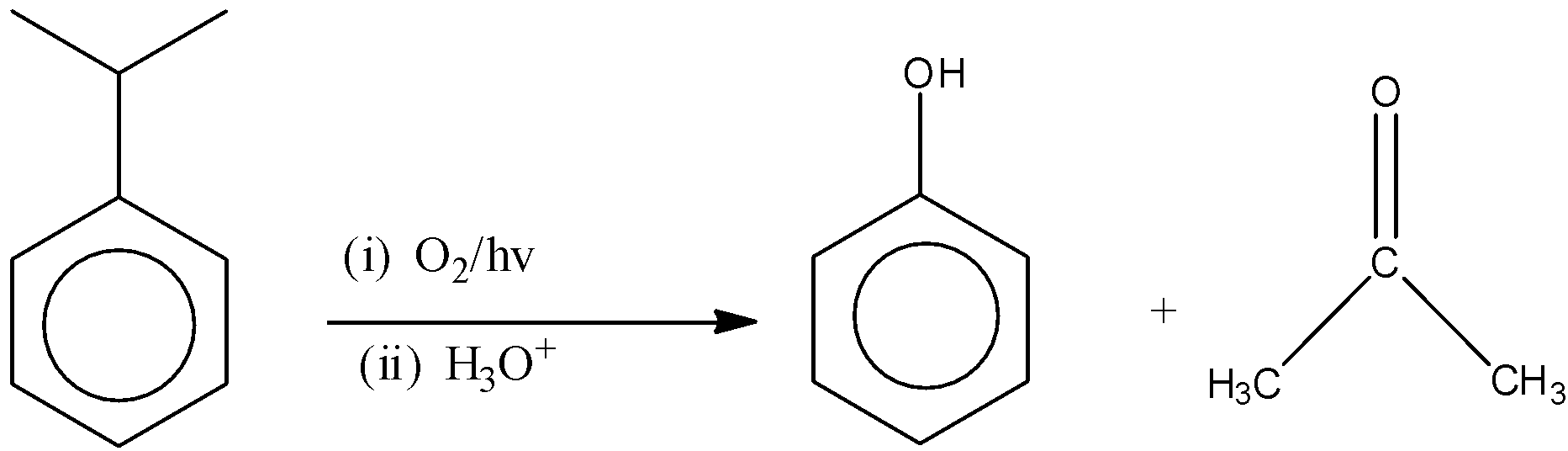
(C)
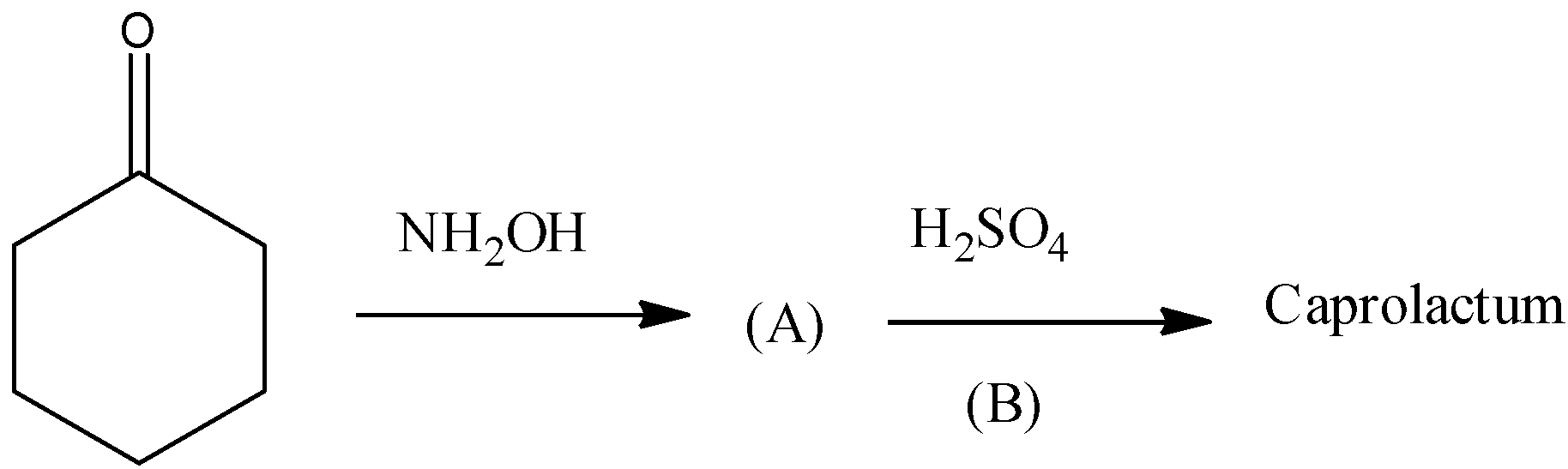
(D)
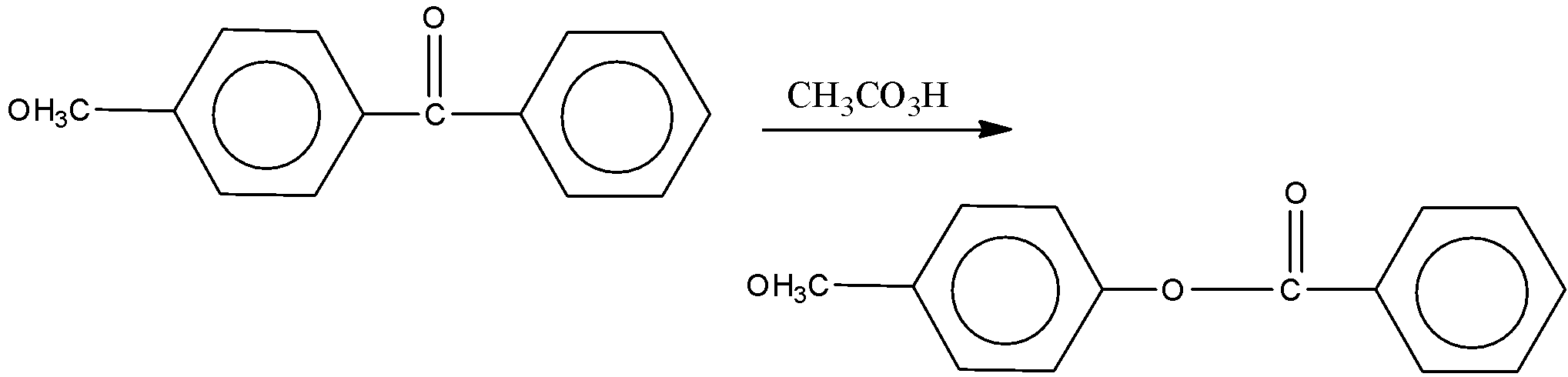
Solution
We know that amides are the class of compounds in which one atom of ammonia is replaced by a carboxyl group. Hoffmann bromamide reaction is one of the important reactions of amide used to prepare primary amines.
Complete step by step answer:
Now, we discuss the Hoffmann bromamide reaction in detail. In this reaction, amides with no substituent on nitrogen react with solutions of bromine or chlorine in presence of sodium hydroxide to result in amine. In this reaction, the formed amide contains one less carbon atom than the amide.
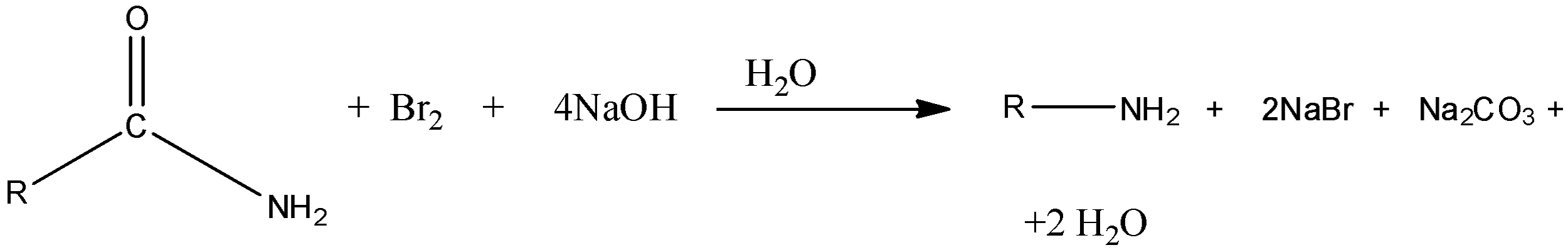
Here, R is an alkyl group.
Now, come to the question. We have to identify incorrect major products formed in the reaction.
In option (A) the following reaction is given.
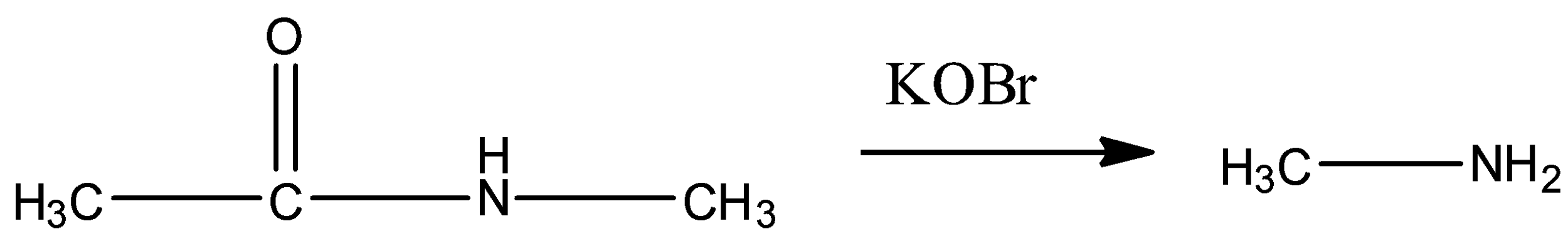
Here, an amide is converted to a primary amine in presence of KOBr. That means, the reaction is a Hoffmann Bromamide reaction. But, we know that to undergo Hofmann Bromamide reaction the amide must not have substituents on the N atom. Here, one methyl group is present as substituents in the N atom. So, conversion of this amide to primary amine is not possible by Hoffmann Bromamide reaction.
So, option A represents an incorrect major product.
Hence, the correct answer is option A.
Note:
Always remember that in Hoffmann bromamide reaction, an aryl or alkyl group migrates from the carbonyl carbon of amide to the nitrogen atom. So, the formed amide contains one less number of carbon atom than the amide. Primary amines formed by this reaction are not contaminated by secondary and tertiary amines.
