Question
Question: Which of the following reactions proceeds with retention of configuration?...
Which of the following reactions proceeds with retention of configuration?
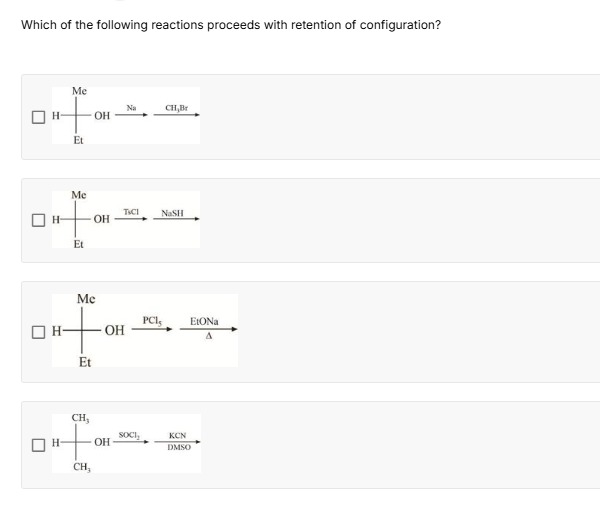
A secondary alcohol reacts with sodium (Na) to form an alkoxide, followed by reaction with methyl bromide (CH3Br).
A secondary alcohol reacts with p-toluenesulfonyl chloride (TsCl) to form a tosylate, followed by reaction with sodium hydrosulfide (NaSH).
A secondary alcohol reacts with phosphorus pentachloride (PCl5) to form an alkyl chloride, followed by reaction with sodium ethoxide (EtONa) and heat (Δ).
Propan-2-ol reacts with SOCl2 to form an alkyl chloride, followed by reaction with KCN in DMSO.
Option 1 and Option 3
Solution
Option 1: The reaction involves a secondary alcohol reacting with sodium (Na) to form an alkoxide, followed by reaction with methyl bromide (CH3Br). This is a Williamson ether synthesis. The alkoxide attacks the methyl carbon of CH3Br in an SN2 reaction. Since the chiral center of the alcohol is not involved in bond breaking or formation, the configuration is retained.
Option 2: The reaction involves a secondary alcohol reacting with p-toluenesulfonyl chloride (TsCl) to form a tosylate, followed by reaction with sodium hydrosulfide (NaSH). The tosylate group is an excellent leaving group, and the reaction with SH− is an SN2 reaction, which proceeds with inversion of configuration.
Option 3: The reaction involves a secondary alcohol reacting with phosphorus pentachloride (PCl5) to form an alkyl chloride, followed by reaction with sodium ethoxide (EtONa) and heat (Δ). The reaction of secondary alcohols with PCl5 generally proceeds with inversion of configuration. The subsequent reaction of the alkyl chloride with sodium ethoxide is an SN2 reaction, which also proceeds with inversion of configuration. A double inversion results in the retention of configuration.
Option 4: The starting material shown is propan-2-ol, which is achiral because the central carbon is bonded to two identical methyl groups. The concept of retention of configuration is not applicable to achiral molecules.
