Question
Question: Which of the following reactions is an example of oxidative decarboxylation? A. Conversion of succ...
Which of the following reactions is an example of oxidative decarboxylation?
A. Conversion of succinate to fumarate.
B. Conversion of fumarate to malate.
C. Conversion of pyruvate to acetyl Co-A.
D. Conversion of citrate to isocitrate.
Solution
Oxidative decarboxylation is a type of oxidative chemical reaction in which carbon dioxide is going to be removed in the form of a gas. Generally this type of chemical reaction is going to occur in living organisms.
Complete answer:
- In the question it is asked to find an example for oxidative decarboxylation reaction among the given options.
- Coming to the given options, option A, Conversion of succinate to fumarate.
- The chemical reaction of Conversion of succinate to fumarate is as follows.
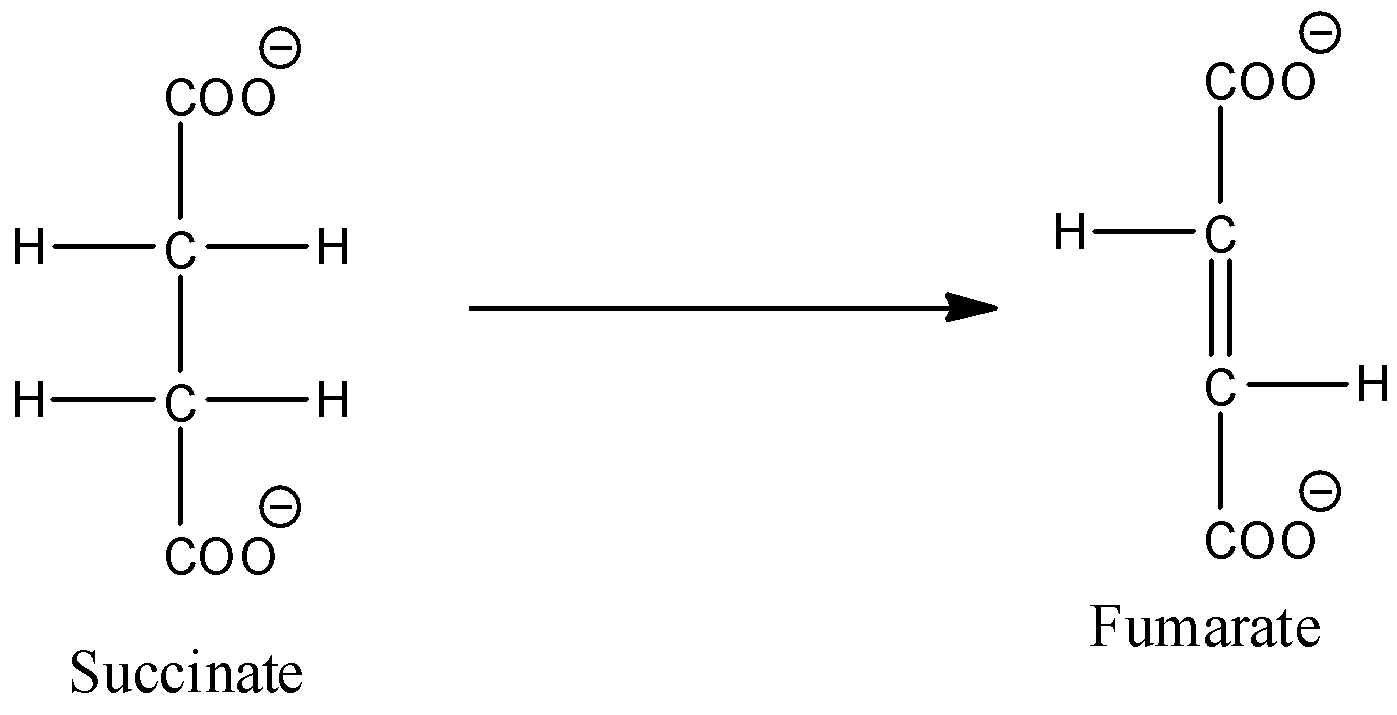
- In the above chemical reaction we can see that the Conversion of succinate to fumarate is an example for oxidation.
- Therefore option A is wrong.
- Coming to option B, Conversion of fumarate to malate.
- The chemical reaction which represents Conversion of fumarate to malate is as follows.
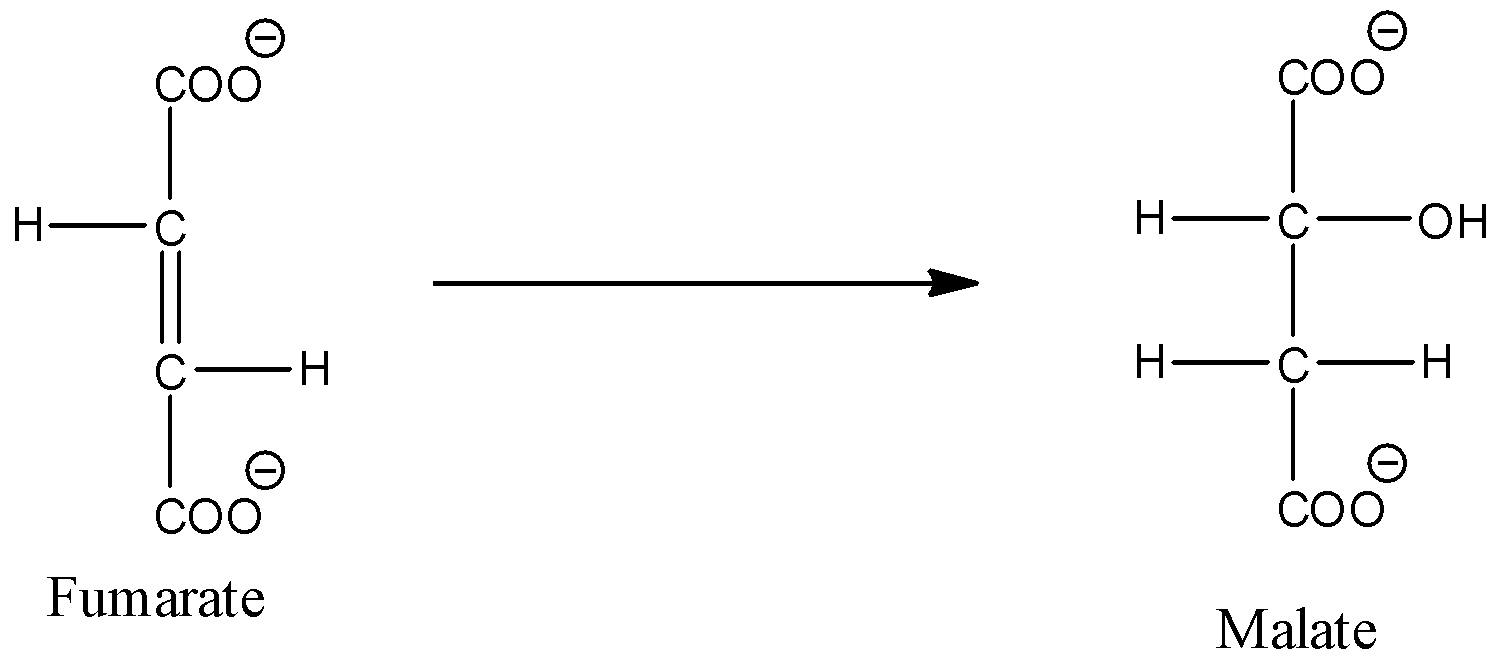
- In the above chemical reaction we can see that fumarate is going to convert into malate by hydrolysis reaction.
- Therefore option B is incorrect.
- Coming to option C, Conversion of pyruvate to acetyl Co-A.
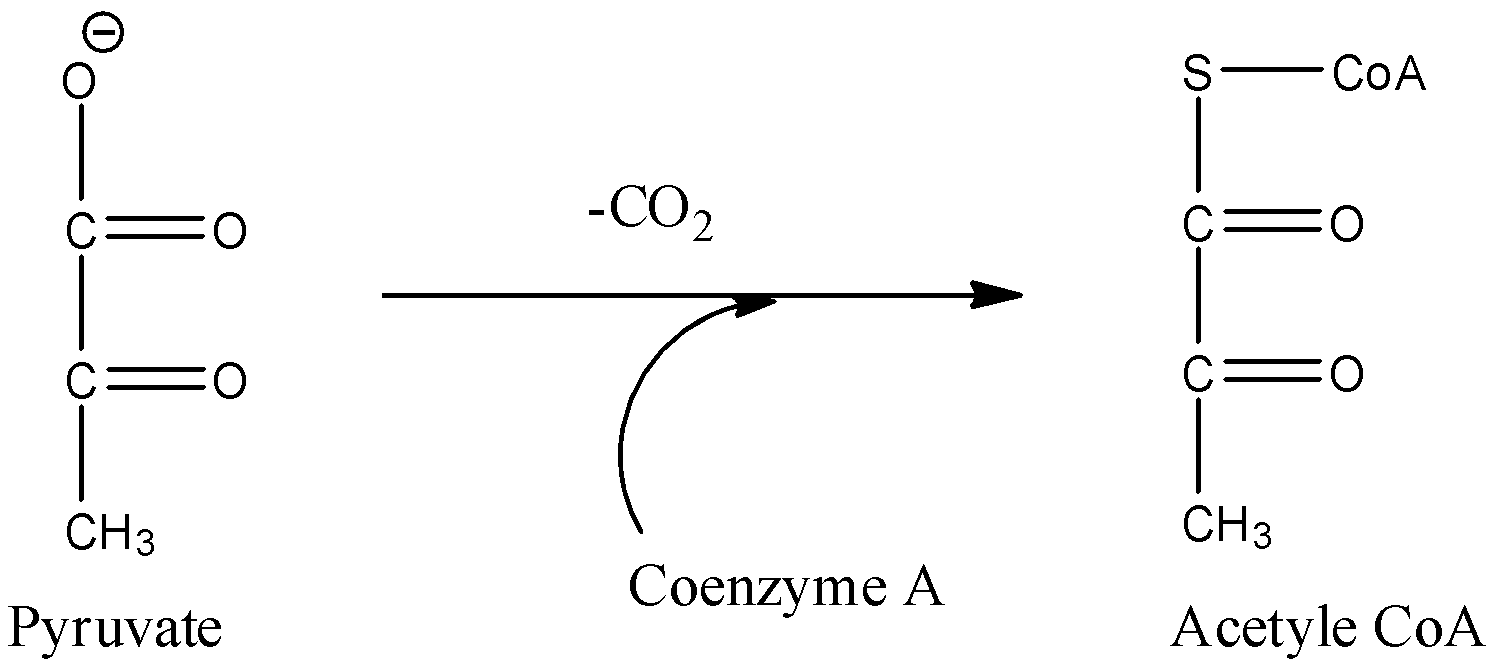
- In the above chemical reaction we can see that decarboxylation is occurring at the same time oxidation is also occurring.
- Therefore the option C is correct.
- Coming to the option D, Conversion of citrate to isocitrate.
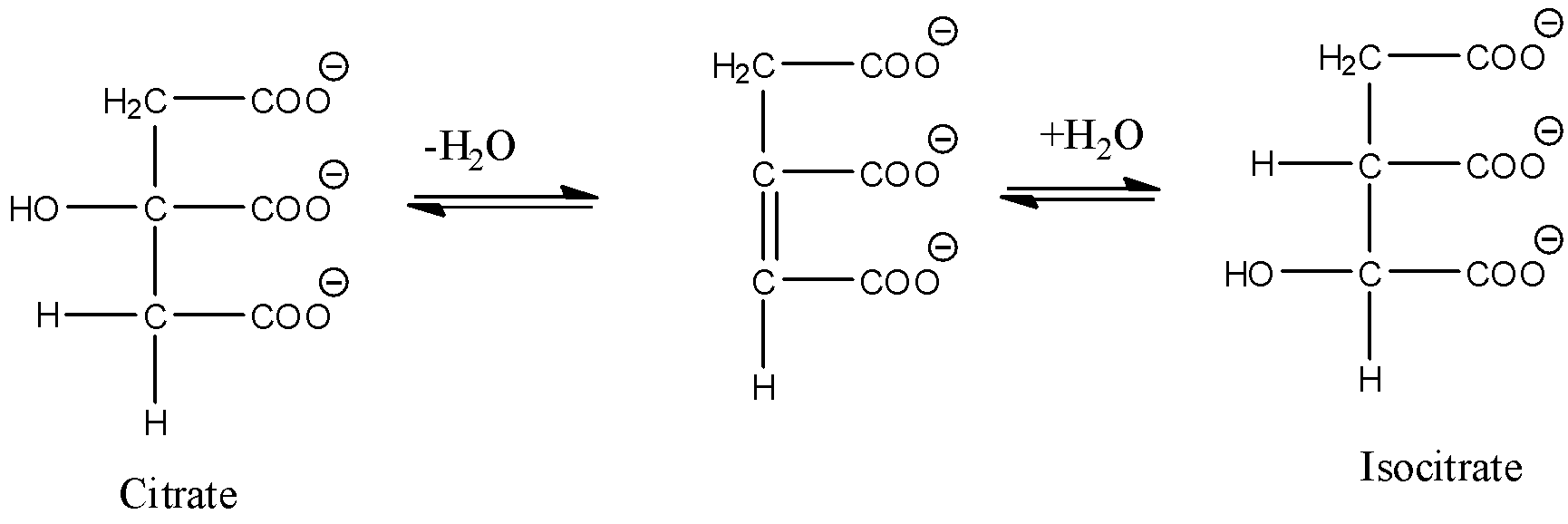
- The above reaction is an example for dehydration and hydration.
Therefore the correct option for oxidative decarboxylation is C.
Note: The pyruvate forms from the glucose and enters the cycle to convert into acetyl Co-A by an enzyme called coenzyme A. The pyruvate is the last product in the process of glycolysis to produce energy.
