Question
Question: Which of the following reactions are examples of chemisorption? A.Haber Process B.Activated cha...
Which of the following reactions are examples of chemisorption?
A.Haber Process
B.Activated charcoal to decolorize sugar
C.Cross coupling reactions
D.Hydrogenation of Vanaspati ghee
Solution
Chemisorption is a type of adsorption that involves a chemical reaction between the surface and the adsorbate. This means that the new chemical bonds are generated at the surface.
Complete step by step answer:
Adsorption is a process in which there is the accumulation of a substance in molecular species in higher concentration on the surface .
There are two types of adsorption on the basis of interaction forces between adsorbate and adsorbent.
-Physical adsorption
-Chemical adsorption
Physical Adsorption:
It is also known as physisorption. It occurs generally due to the weak van der waals forces present between adsorbate and adsorbent.
For example: H2 and N2 adsorb on coconut charcoal.
Chemical Adsorption:
It is also known as chemisorption. It occurs due to the strong chemical forces of bonding between adsorbate and adsorbent.
From the examples given in the question, the Haber process and hydrogenation of vanaspati ghee are the examples of chemisorption.
Haber process: In this process, nitrogen combines from the air with hydrogen derived mainly from methane (natural gas) into ammonia.
The reaction equation of haber's process is:
N2(g)+3H2(g)⇌2NH3(g)
Hydrogenation of vanaspati ghee: This is known to be a chemical reaction between molecular hydrogen (H2) and another element in the presence of catalysts such as nickel, palladium or platinum.
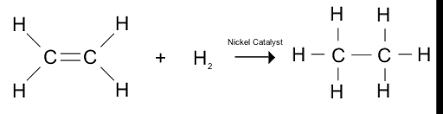
This is the reaction of hydrogenation of vanaspati ghee.
Therefore, option A and D are the answers.
Note:
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent.
