Question
Question: Which of the following reaction is not feasible...
Which of the following reaction is not feasible
A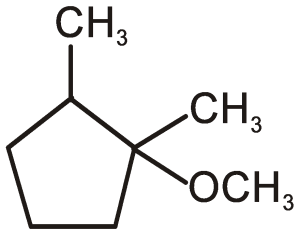
 CH3OHΔ
CH3OHΔ +
+

B
 C2H5OHΔ
C2H5OHΔ +
+

C


 +
+
 +
+ 
D


 + HCl + I⊖
+ HCl + I⊖
Answer


 + HCl + I⊖
+ HCl + I⊖
Explanation
Solution
In aryl halides the C – X bond has partial double bond character due to resonance so the cleavage of C – X bond becomes difficult.



