Question
Question: Which of the following reaction is **incorrect**?...
Which of the following reaction is incorrect?
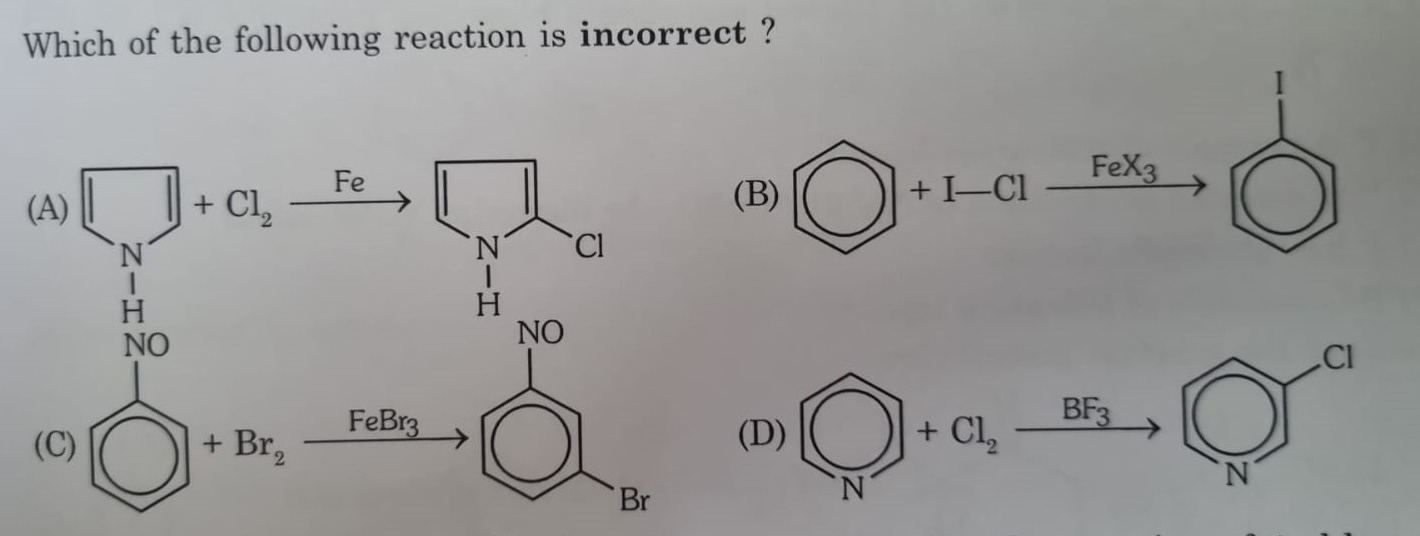
Reaction of N-(4-nitrophenyl)pyrrole with Cl2 in the presence of Fe.
Reaction of benzene with I-Cl in the presence of FeX3.
Reaction of nitrobenzene with Br2 in the presence of FeBr3.
Reaction of pyridine with Cl2 in the presence of BF3.
Reaction of pyridine with Cl2 in the presence of BF3.
Solution
The given options show various reactions involving aromatic and heterocyclic compounds undergoing substitution reactions. Let's analyze each reaction:
(A) Reaction of N-(4-nitrophenyl)pyrrole with Cl2 in the presence of Fe. This is an electrophilic substitution reaction. Pyrrole is an electron-rich heterocyclic compound and is highly reactive towards electrophilic substitution. The positions 2 and 5 are most reactive. The product shown is 2-chlorinated pyrrole derivative. This reaction is plausible.
(B) Reaction of benzene with I-Cl in the presence of FeX3. This is an electrophilic aromatic substitution. In the presence of a Lewis acid like FeX3, I-Cl can generate I+ electrophile. Benzene undergoes electrophilic substitution to form iodobenzene. This reaction is correct.
(C) Reaction of nitrobenzene with Br2 in the presence of FeBr3. This is an electrophilic aromatic substitution. Nitrobenzene is a deactivated aromatic ring due to the electron-withdrawing nitro group, which is a meta-director. Therefore, electrophilic substitution occurs at the meta position. The product shown is meta-bromonitrobenzene. This reaction is correct.
(D) Reaction of pyridine with Cl2 in the presence of BF3. This is intended to be an electrophilic aromatic substitution. Pyridine is a pi-deficient aromatic system and is deactivated towards electrophilic substitution. The nitrogen atom is electronegative and withdraws electron density, especially from the ortho and para positions. Electrophilic attack is preferred at the meta position (3 and 5). Also, pyridine is a base and forms a complex with the Lewis acid BF3, which further deactivates the ring. Electrophilic substitution on pyridine is difficult and usually requires harsh conditions. When it occurs, the major product is typically the 3-substituted pyridine. The reaction shown forms 2-chloropyridine. Electrophilic substitution at the 2-position of pyridine is disfavored compared to the 3-position under typical conditions. Therefore, this reaction is incorrect.
