Question
Question: Which of the following products is not possible when cyclohexene reacts with bromine in the presence...
Which of the following products is not possible when cyclohexene reacts with bromine in the presence of brine?
(A)
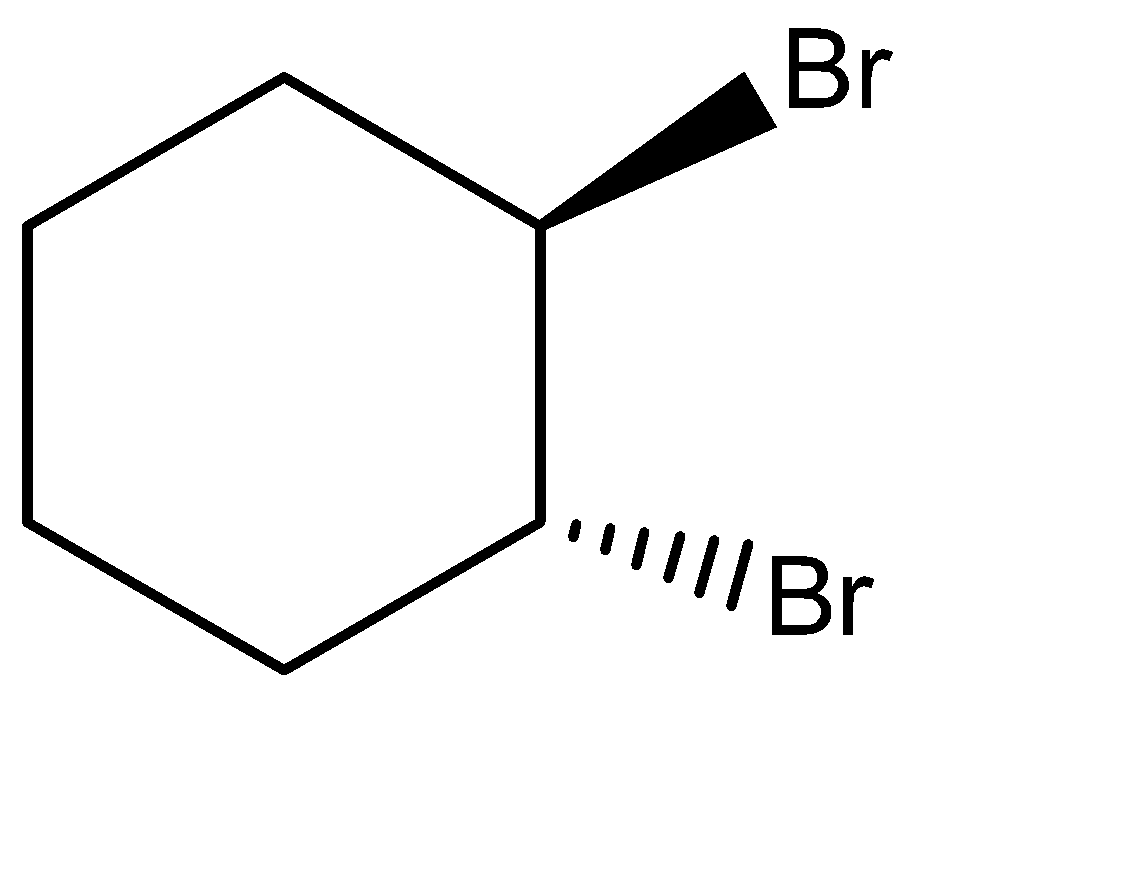
(B)
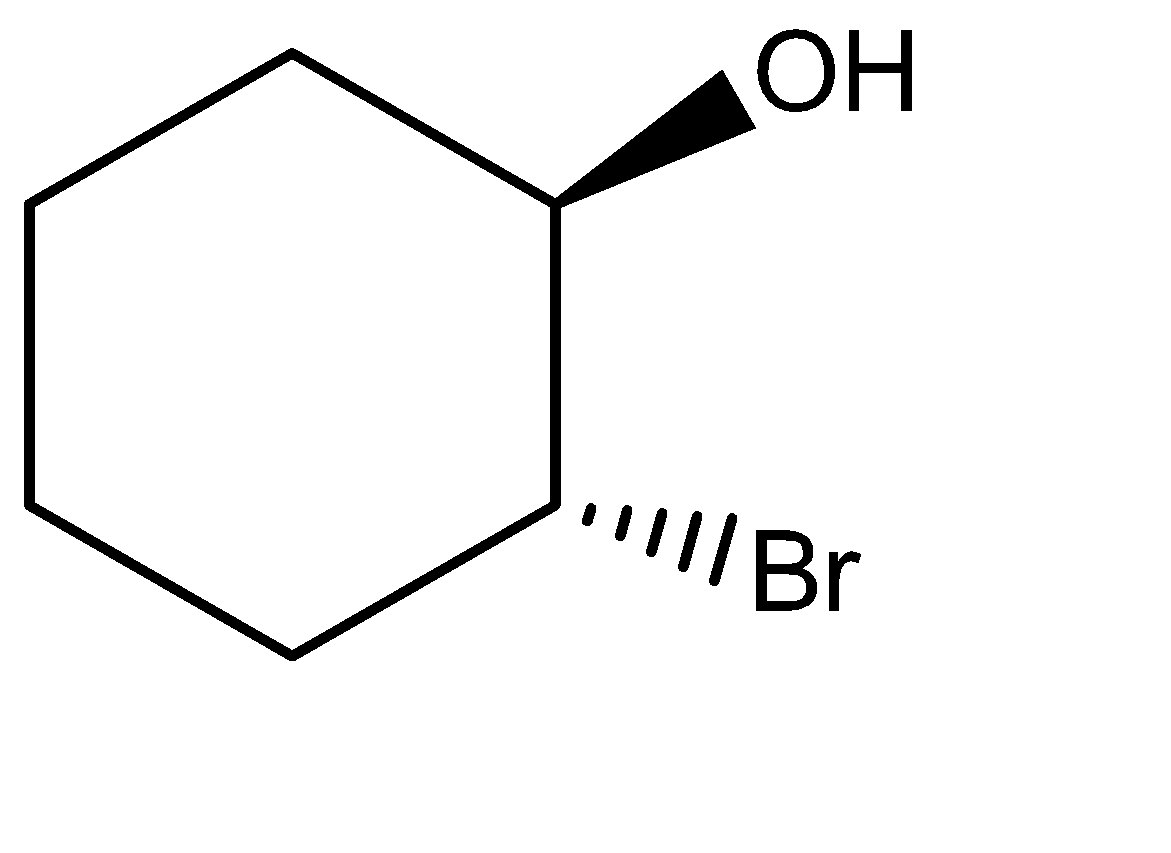
(C)
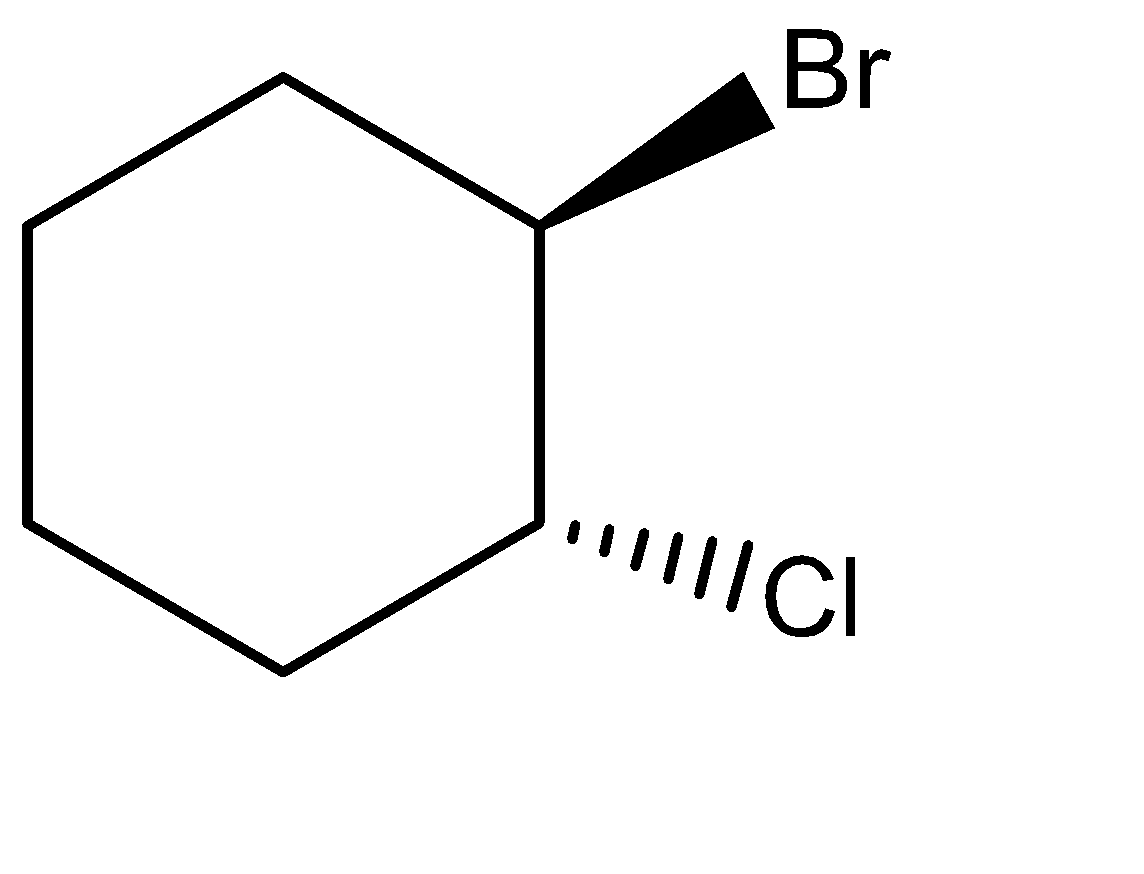
(D)
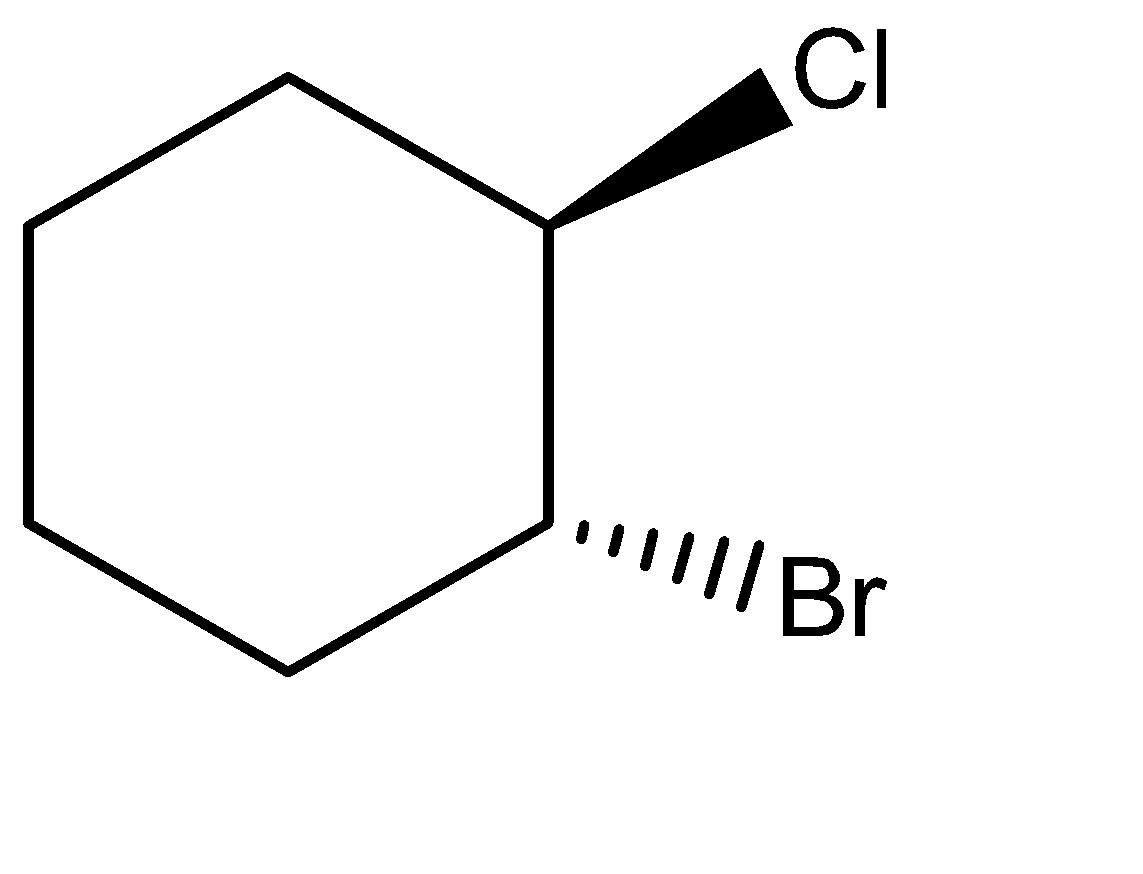
Solution
As we know that brine is the other name for aqueous NaCl solution (sodium chloride mixed in water which produces ions). When an alkene reacts with bromine, the double bond breaks and bromine attach itself to the hydrocarbon. So here we have to talk about the product which is not possible when cyclohexene reacts with bromine in the presence of brine.
Complete answer: Let us first discuss about attack of bromine on cyclohexene followed by the possible products with all the available nucleophiles as follows:-
-As we know that the alkene part of cyclohexene will act as a nucleophile and bromine will act as an electrophile. The bromine part attacks the nucleophile and attaches itself to one side of the double bond. A carbocation is formed as an intermediate. This is a non-classical carbocation as it is formed between three atoms of the ring as shown in the reaction:-
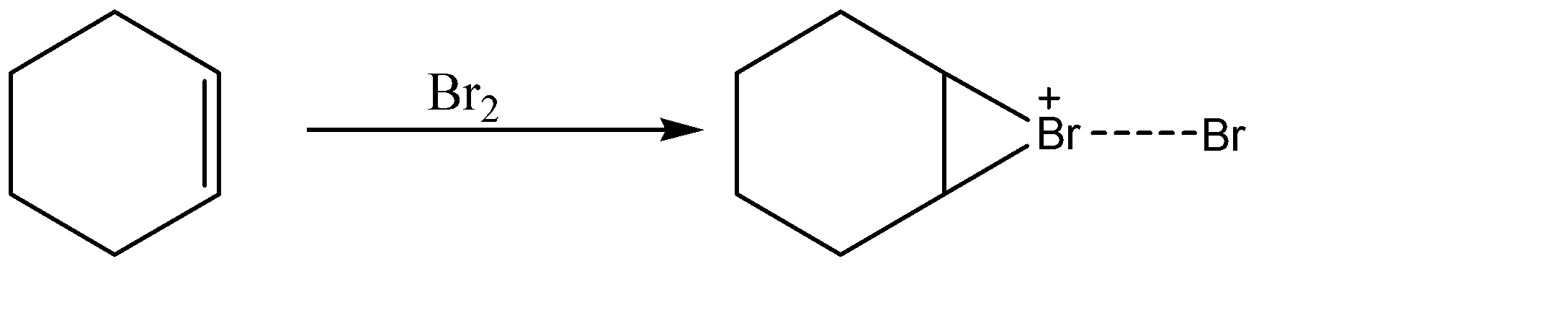
-Now we have 3 nucleophiles in the brine solution with bromine. These nucleophiles are OH−, Cl− and Br−. The bromine anion is in interaction with the non-classical carbocation as it is still loosely attached to the bromine atom involved in the ring. Also it is a weak nucleophile comparatively.
-Attack of chlorine ion on the carbocation:-
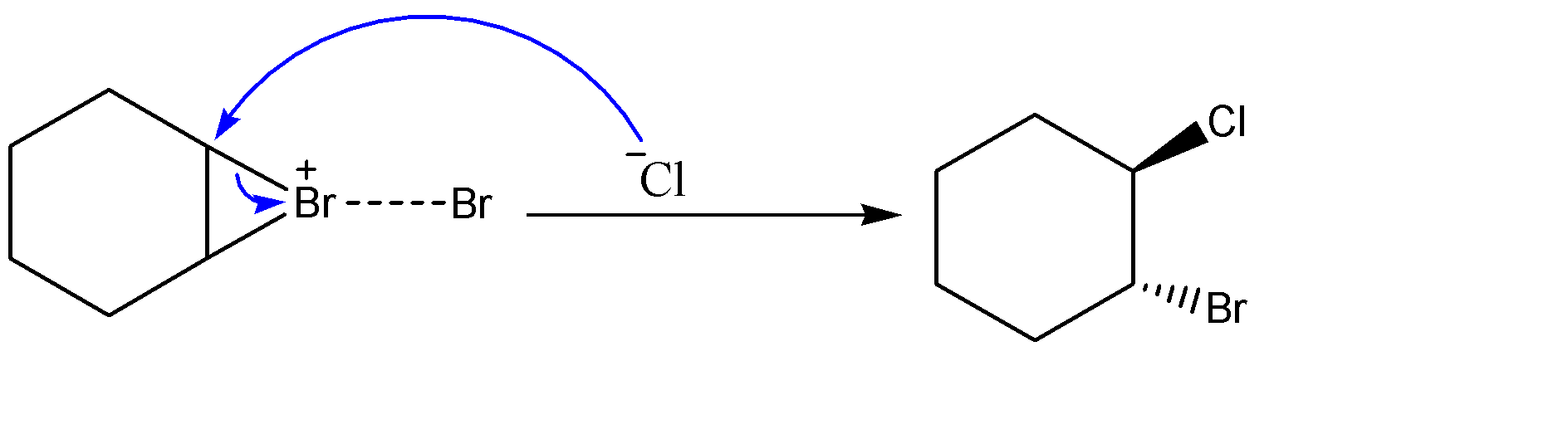
The chlorine ion readily interacts with the electrophilic carbon of the ring. It attacks from the opposite side of the ring to avoid any chances of steric hindrance, hence forming the product.
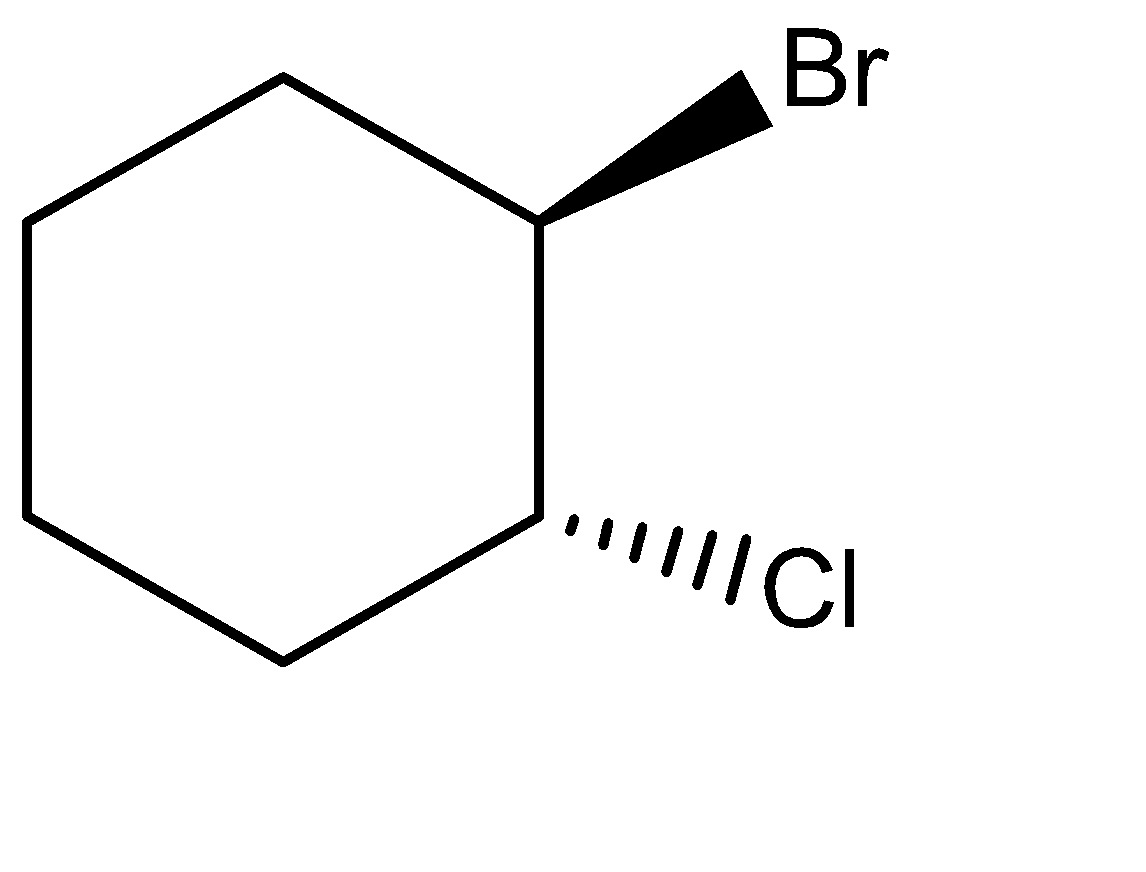
If we rotate the above product by 180∘out of the plane, then it forms the above structure.
-Attack of hydroxide ion on the carbocation:-
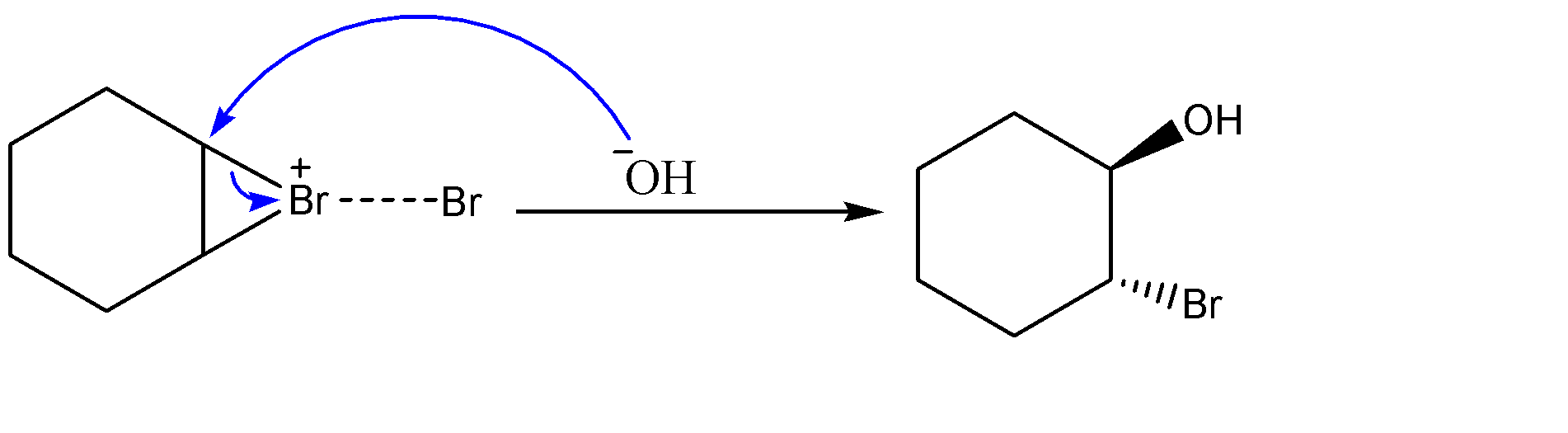
-Similar happens in the case of hydroxide ion when it attacks the non-classical carbocation and forms the above product. Hydroxide ion is quite a good nucleophile and acts faster than both chlorine and bromine ions as well.
Therefore the product which is not possible when cyclohexene reacts with bromine in the presence of brine is (A)
Note:
-Remember that bromine ion is a weak nucleophile as compared to chlorine and hydroxide ions because it is large in size and has comparatively less mobility.
-Also non-classical cations are completely different from classical cations (positive charge lies on one atom) and do not show any of its properties.
