Question
Question: Which of the following product is formed in the below reaction: 
A.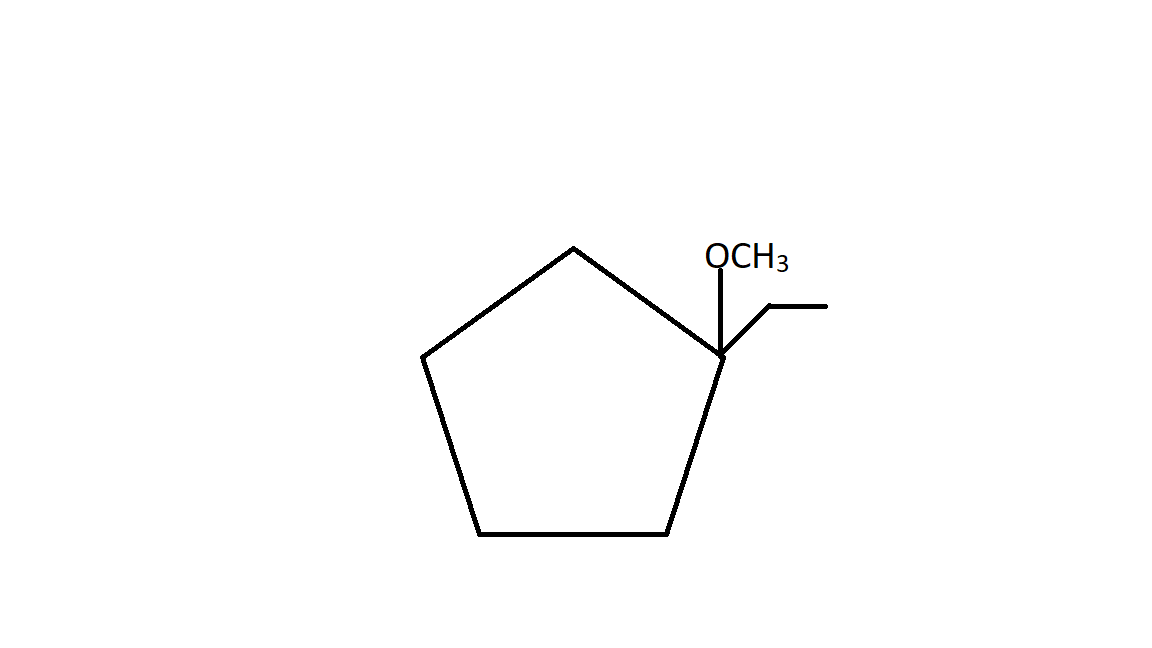
B.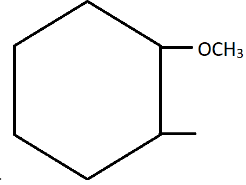
C.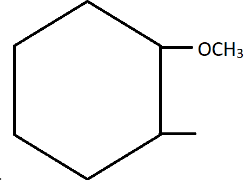
D.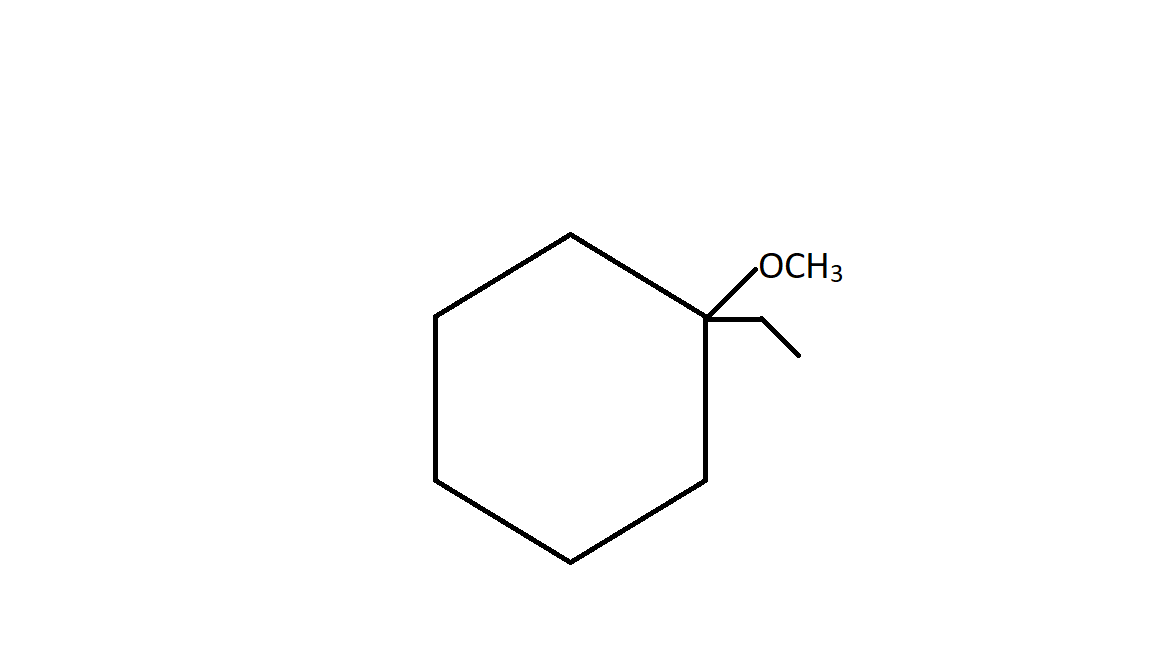
Solution
Addition of alkene is an electrophilic addition reaction in which there is a formation of carbocation [C+]. Rearrange the cation to that carbon where it is most stable. If ring expansion takes place then make a ring of 6 carbon atoms. Six carbon rings are the most stable cycloalkane.
Complete answer:
Addition of nucleophile and takes place through the bond breaking process. Once the pi bond of alkene breaks, then electrophile attacks on the compound.
1. Breaking of pi bond.
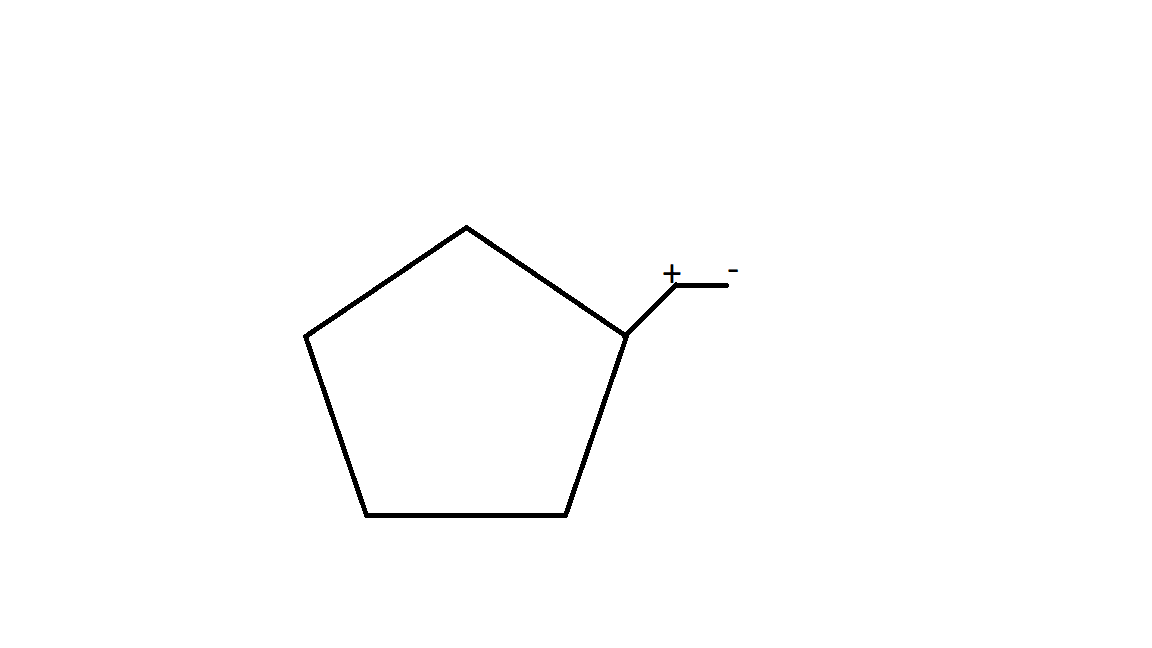
2.Now the carbocation formed is not stable at 2∘ carbocation. Reason for the stability of carbocation depends on the inductive effect of the alkyl group. 3∘ carbocation is the most stable carbocation.
While moving the carbocation, ring expansion takes place.
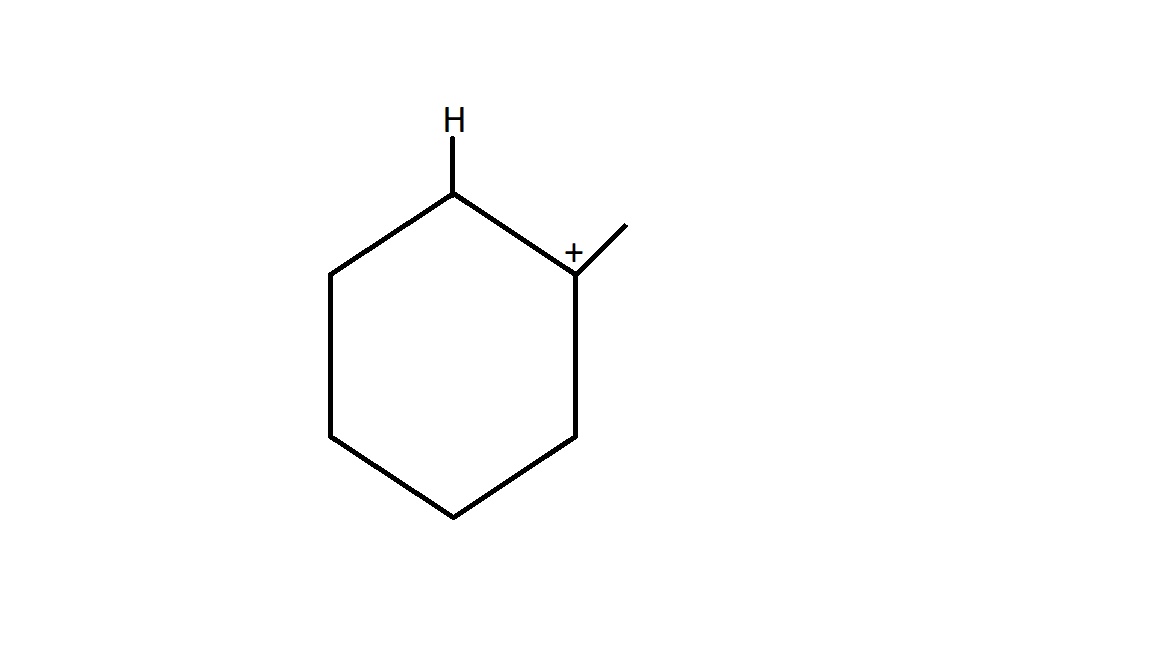
3. Generation of nucleophiles .
CH3OH CH3O− + H+
Now the nucleophile will attack at carbocation. Here the degree of carbocation is 3∙, which is the most stable form of carbocation.
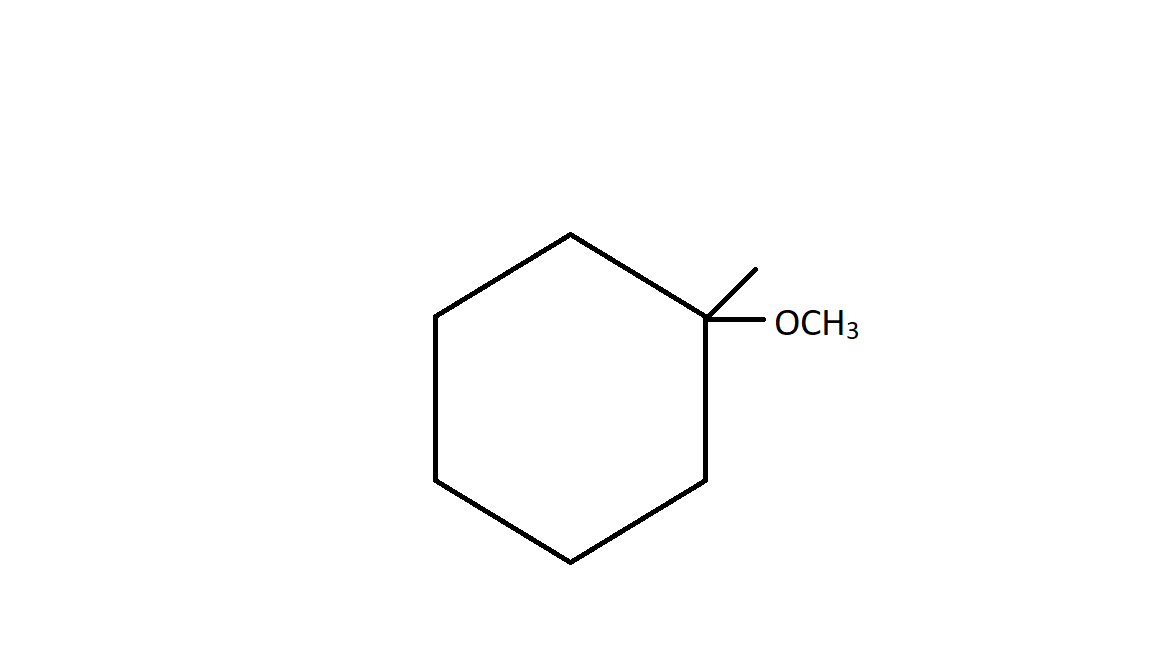
Hence after rearrangement of carbocation and ring expansion the product formed is a six member ring which is more stable than a five member ring. Therefore nucleophile attack on 3∙carbocation and thus giving the above structure.
Hence the correct answer is option C.
Additional Information:
The order of stability of carbocation is:
The least stable carbocation is 1∙.
Cyclohexane is more stable than cyclopentane because of lesser angle strain.
Note:
While doing ring expansion make sure the number of carbon in the ring does not exceed more than six. After six, the stability of the compound decreases. The stability of carbocation must be satisfied while performing ring expansion. Nucleophile attacks at the last stage of reaction.
