Question
Question: Which of the following processes is (are exothermic)? ?
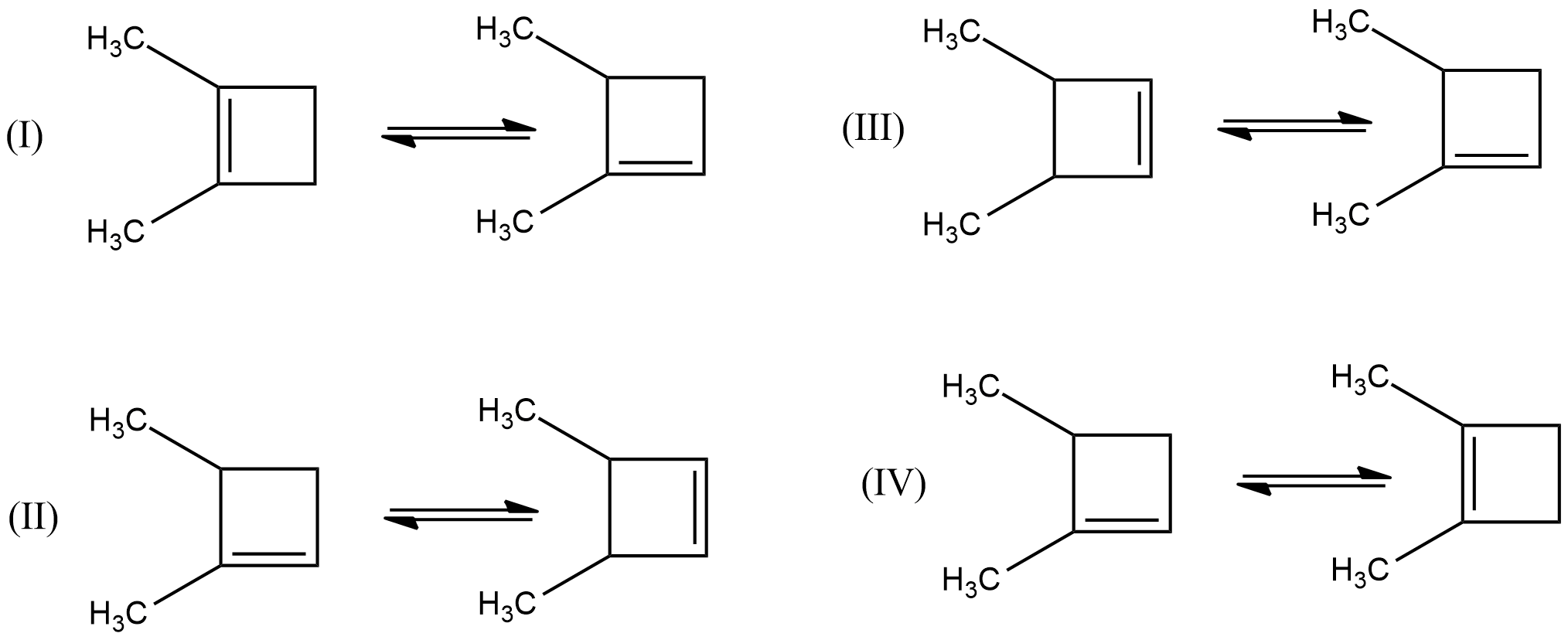
A. All of these
B. (I) and (II) only
C. (III) and (IV) only
D. (I), (II), and (IV) only
Solution
The chemical reaction in which if energy is going to be released then the chemical reaction is called exothermic chemical reaction. The chemical reaction in which the energy is going to be absorbed then the chemical reaction is called endothermic chemical reaction.
Complete answer:
- In the question it is asked to find the chemical reaction in which an exothermic reaction is going to take place.
- Before going to discuss the given chemical reactions we should know that in the chemical reaction the double bond is going to rearrange and form a structure with highly substituted carbon then the reaction is called exothermic reactions.
- Because at the time of the rearrangement of the double bond towards the highly substituted carbon there is a release of the energy that is going to take place and makes the molecule to get less energy and more stable.
- Means we have to find the chemical reaction in which the rearrangement of the double bond is going to occur towards the highly substituted carbon.
- Among the given chemical reactions, we can easily see that in the chemical reactions (III) and (IV) the double bond is going to rearrange towards the highly substituted carbon and the chemical reactions are as follows.
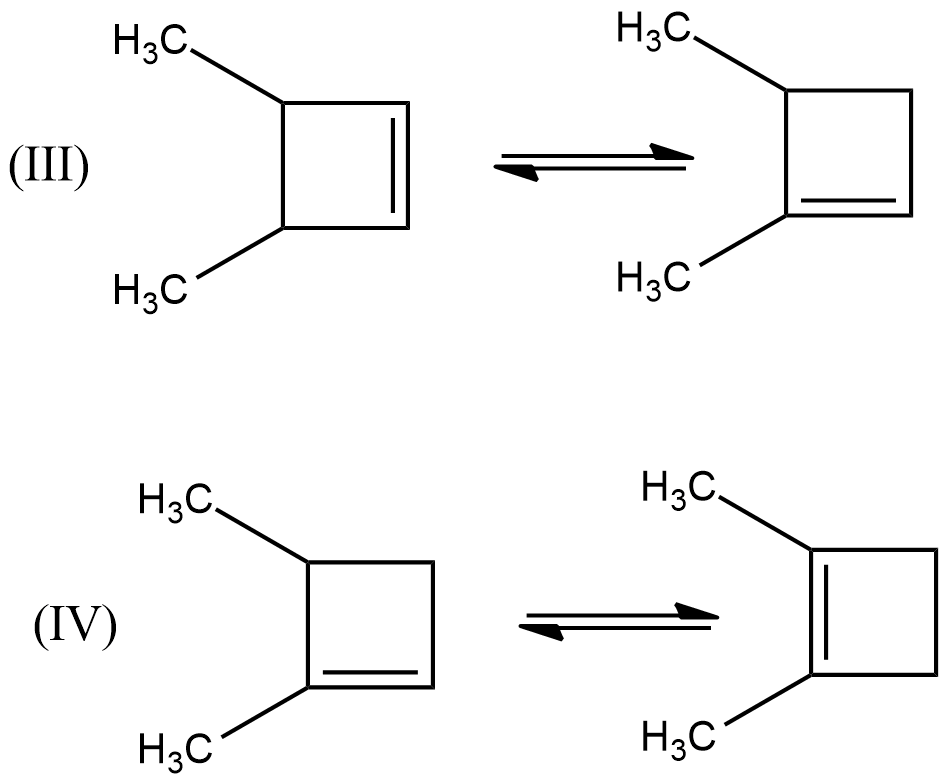
- Therefore, the chemical reactions which are going to release the energy means exothermic reactions are (III) and (IV).
So, the correct option is C.
Note:
The compounds which have highly substituted carbons are highly stable in nature due to the presence of the less energy with them. The enthalpy of the highly substituted compounds is very less when compared to less substituted compounds.
