Question
Question: Which of the following plots will be obtained for a conductometric titration of strong acid against ...
Which of the following plots will be obtained for a conductometric titration of strong acid against a weak base?
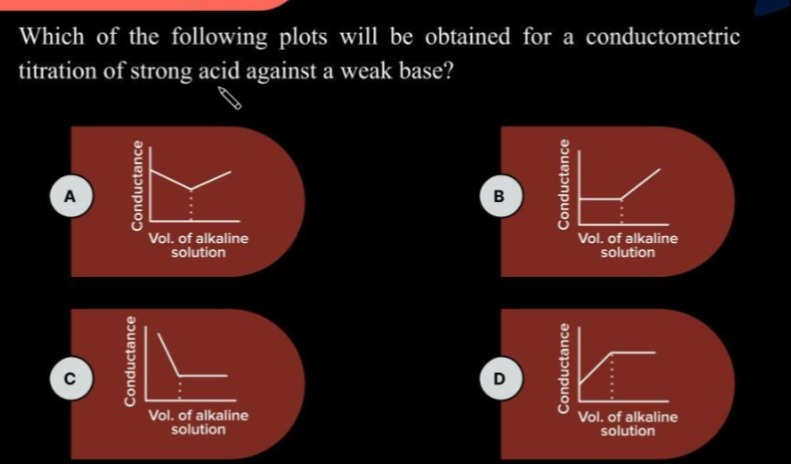
A
Solution
The conductometric titration of a strong acid against a weak base involves the reaction between the strong acid and the weak base. Let's consider the titration of HCl (strong acid) with NH4OH (weak base). The reaction is:
HCl + NH4OH → NH4Cl + H2O
Initially, the solution contains a strong acid, which is completely dissociated into H+ and Cl− ions. The conductance is high due to the presence of highly mobile H+ ions.
As the weak base (NH4OH) is added, it reacts with the H+ ions to form water and the salt NH4Cl.
H+ + Cl− + NH4OH → NH4+ + Cl− + H2O
The highly mobile H+ ions are removed from the solution and are replaced by less mobile NH4+ ions. The concentration of H+ decreases, while the concentration of NH4+ increases. The concentration of Cl− changes due to volume increase, but the number of Cl− ions remains constant. Since the molar conductivity of H+ ions is much higher than that of NH4+ ions, the conductance of the solution decreases as the titration proceeds before the equivalence point.
At the equivalence point, all the strong acid has been neutralized. The solution contains mainly the salt NH4Cl. The conductance at the equivalence point is due to the ions of the salt.
After the equivalence point, excess weak base (NH4OH) is added. Since NH4OH is a weak base, it is only slightly dissociated in water.
NH4OH ⇌ NH4+ + OH−
The addition of the weakly dissociated weak base increases the volume of the solution and adds a small number of ions (NH4+ and OH−). Therefore, the conductance of the solution increases slightly after the equivalence point. The increase is not as steep as the decrease before the equivalence point because the concentration of ions added is relatively low due to the weak dissociation of the base.
Thus, the conductometric titration curve for a strong acid against a weak base shows an initial decrease in conductance, a minimum at the equivalence point, and a slight increase in conductance after the equivalence point. This corresponds to the shape shown in Option A.
