Question
Question: Which of the following pairs of d-orbitals will have electron density along the axis. A. \({{\text...
Which of the following pairs of d-orbitals will have electron density along the axis.
A. dz2,dx2−y2
B. dxy,dx2−y2
C. dz2,dxz
D. dxz,dyz
Solution
The dz2 and dx2−y2 orbitals which lies on the axis and the dxz,dyz, and dxy lies in between the axis. Total five orbitals are present in the d - orbitals.
Complete step by step answer:
The position of electron density depends upon the position of the orbitals.
The d-orbital is a set of five denigrate orbitals named as dz2, dx2−y2, dxz,dyz, and dxy.
The positions of these five d-orbitals on the axis is represented as follows:
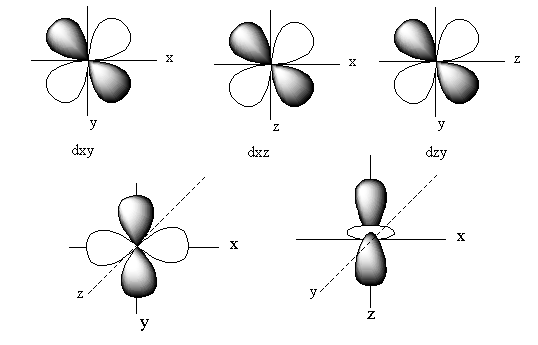
The orbitals of dxy lie in between the x and y-axis. So, the electron density of the dxy lies in between the x and y-axis.
The orbitals of dyz lie in between the z and y-axis. So, the electron density of the dyz lies in between the z and y-axis.
The orbitals of dxz lie in between the x and z-axis. So, the electron density of the dxz lies in between the x and z-axis.
The orbital of dz2lies on the z-axis. So, the electron density of the dz2lies on the z-axis.
The orbitals of dx2−y2 lie on the x and y-axis. So, the electron density of the dx2−y2 lies on the x and y-axis.
So, dz2,dx2−y2 pairs of d-orbitals will have electron density along the axis.
Therefore, option (A) dz2,dx2−y2 is correct.
Note: Out of five d-orbitals, two lie on the axis and three lie in between the axis. So, when ligands form a complex with the metal it affects the three orbitals differently and two orbitals differently, so the five d-orbitals break into two parts of three and two.
