Question
Question: Which of the following pairs of compounds are positional isomers? A) \(C{H_3} - C{H_2} - C{H_2} - ...
Which of the following pairs of compounds are positional isomers?
A) CH3−CH2−CH2−CO−CH3 and CH3−CH2−CO−CH2−CH3
B) CH3−CH2−CH2−CO−CH3 and CH3−CH(CH3)−CH2−CHO
C) CH3−CH2−CO−CH2−CH3 and (CH3)2−CH−CH2−CHO
D) CH3−CH2−CH2−CH2−CHO and CH3−CH2−CH2−CO−CH3
Solution
We know the molecules which have the same molecular formula but different arrangements of atoms are called isomers. There are two types of isomers,
1.Structural isomers
2.Stereoisomers
We have to remember that molecules with the same chemical formulas but differ structurally in the sequence in which the atoms are linked are called structural or constitutional isomers whereas stereoisomers are the isomers that have the same molecular formula but differ in spatial arrangement of atoms.
Complete step by step answer:
We can classify the stereoisomer into three categories,
1.Chain isomerism
2.Position isomerism
3.Functional group isomerism
Chain isomerism:
We must remember that in chain isomerism the skeletons are reordered to create different structures.
Position isomerism:
We have to know that in position isomerism the atom changes the position on the parent atom.
Functional isomerism:
We have to remember that functional isomerism is the structural isomers which have the same molecular formula but the atoms are connected in different ways.
The structure of 2-pentanone and 3-pentanone is,
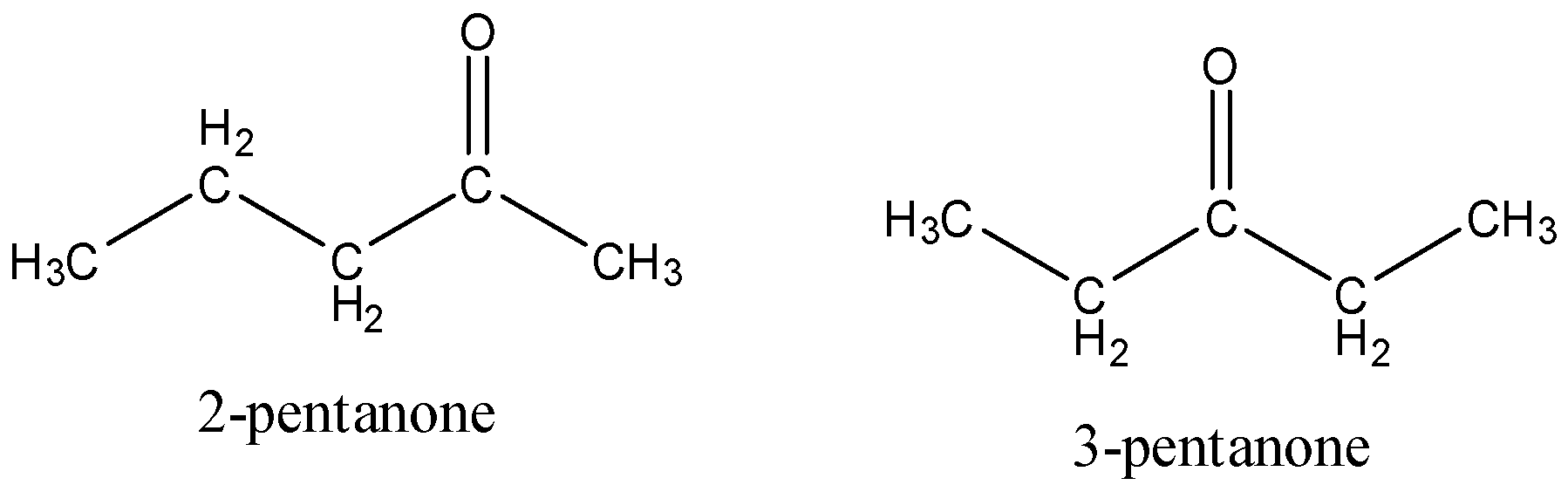
Consequently, 2-pentanone and 3-pentanone in the option A are the position isomers. They vary in the situation of the carbonyl groups.
So, the correct answer is Option A.
Note: Now we can discuss about the concept of stereoisomers as,
As we know that the stereoisomers are the isomers that have the same molecular formula but differ in spatial arrangement of atoms. Stereoisomers can be subdivided into two groups
Optical isomers
1.Geometric isomers
2.Optical isomers:
We have to remember that the optical isomers are the mirror image of a molecule and they can be rotated by plane polarized light.
Geometric isomers:
If there is restricted rotation in a molecule there arises geometric isomerism. Geometric isomers are also known as Cis- Trans isomerism.
If the two atoms locked in same side of the molecule then it is called as cis isomers.
If the two atoms are locked on opposite sides of the molecule then it is called trans isomers.
