Question
Question: Which of the following pairs of compounds are position isomers? A.Isobutyl alcohol and s-butyl al...
Which of the following pairs of compounds are position isomers?
A.Isobutyl alcohol and s-butyl alcohol
B.Isobutyl alcohol and t-butyl alcohol
C.Isobutyl alcohol and neo-pentyl alcohol
D.Ethyl alcohol and ethylene glycol
Solution
We have to know that isomers are two atoms with a similar subatomic recipe however vary fundamentally. In this manner, isomers contain a similar number of iotas for every component, except the nuclear game plan varies. In spite of having a similar subatomic equation, the actual properties of every particle may vary, especially if the practical gatherings related with every atom are unique.
Complete answer:
We have to know that the position of isomers depends on the development of a 'utilitarian gathering' in the atom. A utilitarian gathering in natural science is the piece of a particle that gives it its reactivity. There are a scope of various useful gatherings, the more normal of which were summed up in a past post here. Nothing else about the particle changes basically, where the useful gathering in it is, and the name essentially adjusts somewhat to demonstrate whereabouts in the atom it is found.
For Isobutyl alcohol and s-butyl alcohol, it does not follow the above position of isomers.
Therefore, the option (A) is incorrect.
For Isobutyl alcohol and t-butyl alcohol, it follows the above position isomers.
The structure of the compounds drawn,
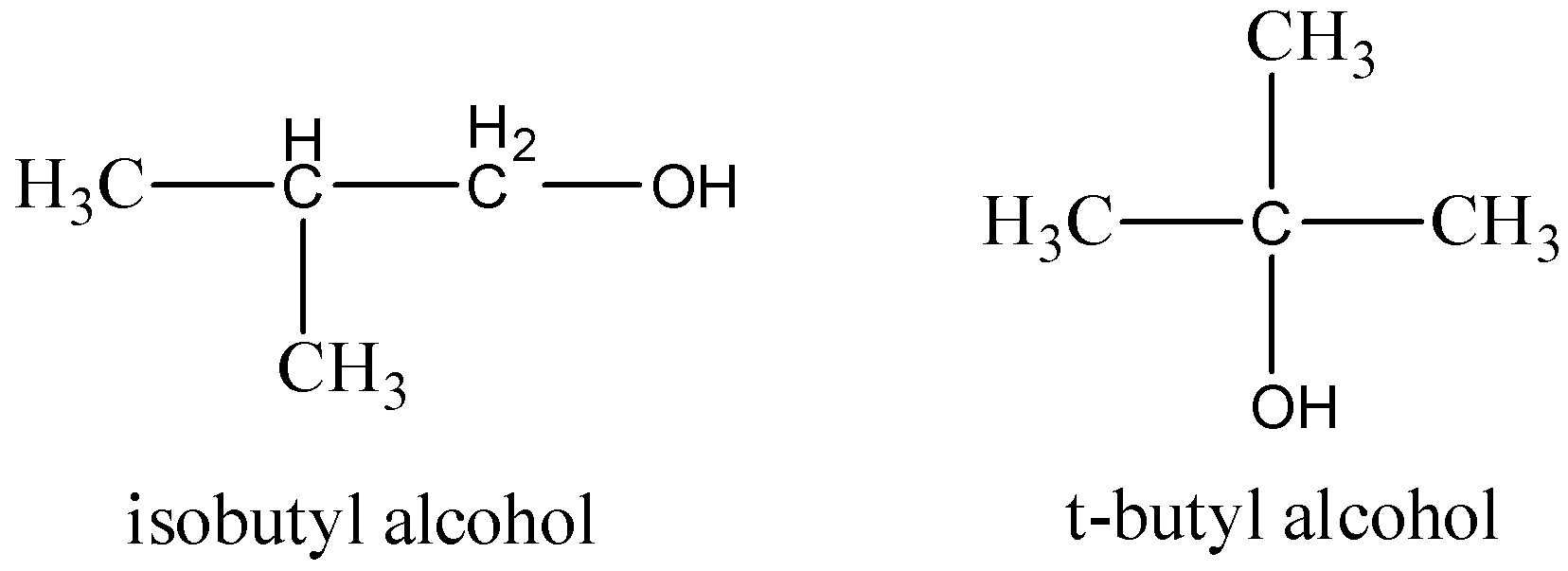
Therefore, the option (B) is correct.
For Isobutyl alcohol and neo-pentyl alcohol, it does not follow the above position of isomers.
Therefore, the option (C) is incorrect.
For ethyl alcohol and ethylene glycol, it does not follow the above position isomers.
Therefore, the option (D) is incorrect.
Note:
We have to know that, the two isomeric mixtures, made out of the very same components in the very same proportion, may have totally different properties in light of the various ways that the molecules are associated inside the mixtures.
