Question
Question: Which of the following pairs are isostructural ? (A) \(SO_4^{2 - }\) and \(BF_4^ - \) (B) \(N{H_...
Which of the following pairs are isostructural ?
(A) SO42− and BF4−
(B) NH3 and NH4+
(C) CO32− and CO2
(D) CH4 and BF3
Solution
Isostructural species are those which have the same shape and hybridization.
Number of election pair =21[N+V−C+A]
Where
V= Number of valence electron present in central atom
N= Number of non-monovalent atoms bonded to covalent atom
C= Charge of cation
A= Charge of anion
Hybridisation can be calculated by using this formula then we will be able to find the structure from hybridisation.
Complete step by step answer:
The given molecules are –
(A) SO42− and BF4−
Number of electron pair in SO42−=21×[6+0+2]=4
Number of electron pairs are 4 that means the hybridisation will be sp3 and the geometry of the molecule will be tetrahedral.
Number of electron pair in BF4−=21×[3+4+1]=4
Number of electron pair are 4 that means the hybridisation will be sp3 and the geometry of molecule will be tetrahedral
Structures
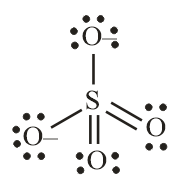
SO42− molecule
(Tetrahedral structure)
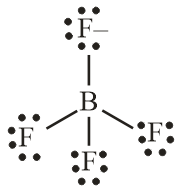
BF4− molecule
(Tetrahedral structure)
(B) NH3 and NH4+
Number of electron pair in NH3=21×[5+3+0]=4
Number of electron pairs are 4 that means the hybridisation will be sp3 and geometry will be tetrahedral. But, In NH3 Nitrogen is surrounded by 3 atoms and the fourth position will be occupied by lone pairs of electrons.
Hence, the structure of NH3 will be pyramidal.
Number of electron pair in NH4+=21[5+4−1]=4
Number of electron pairs are 4 that means the hybridisation will be sp3 and the geometry will be tetrahedral.
Structures
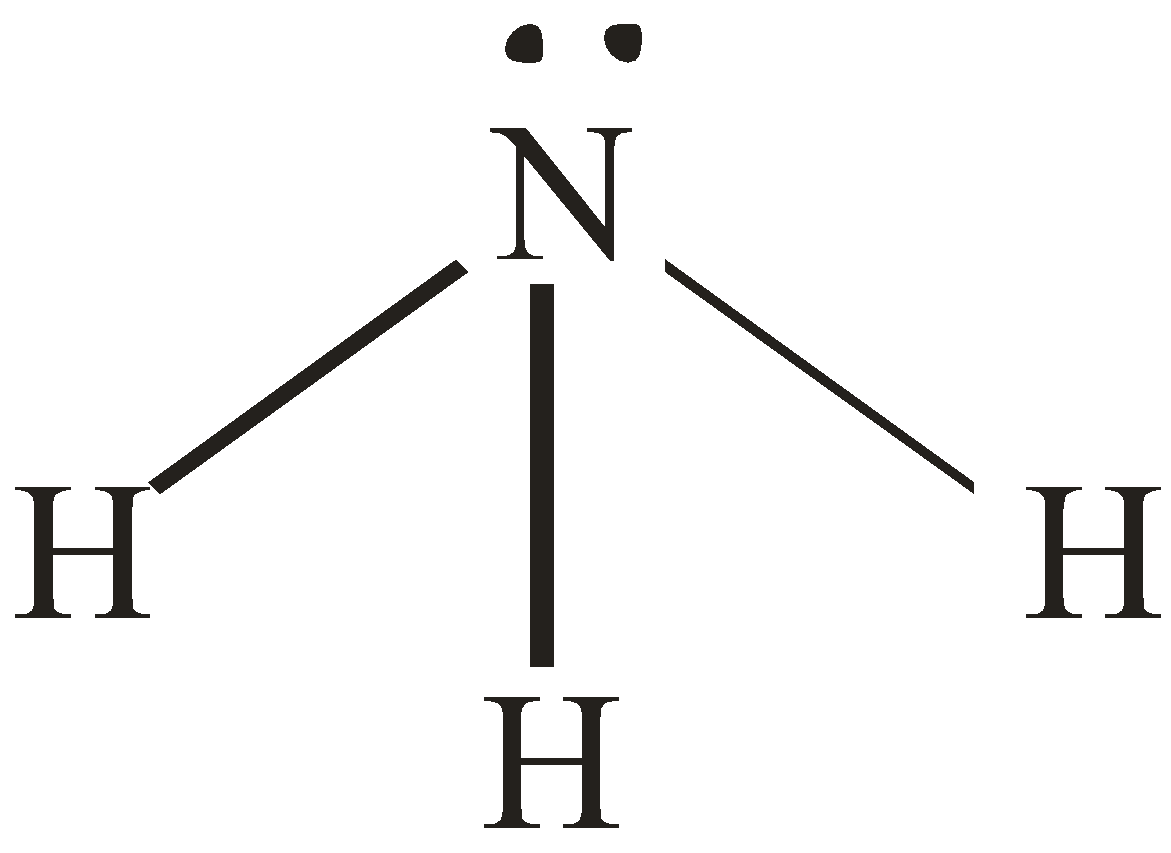
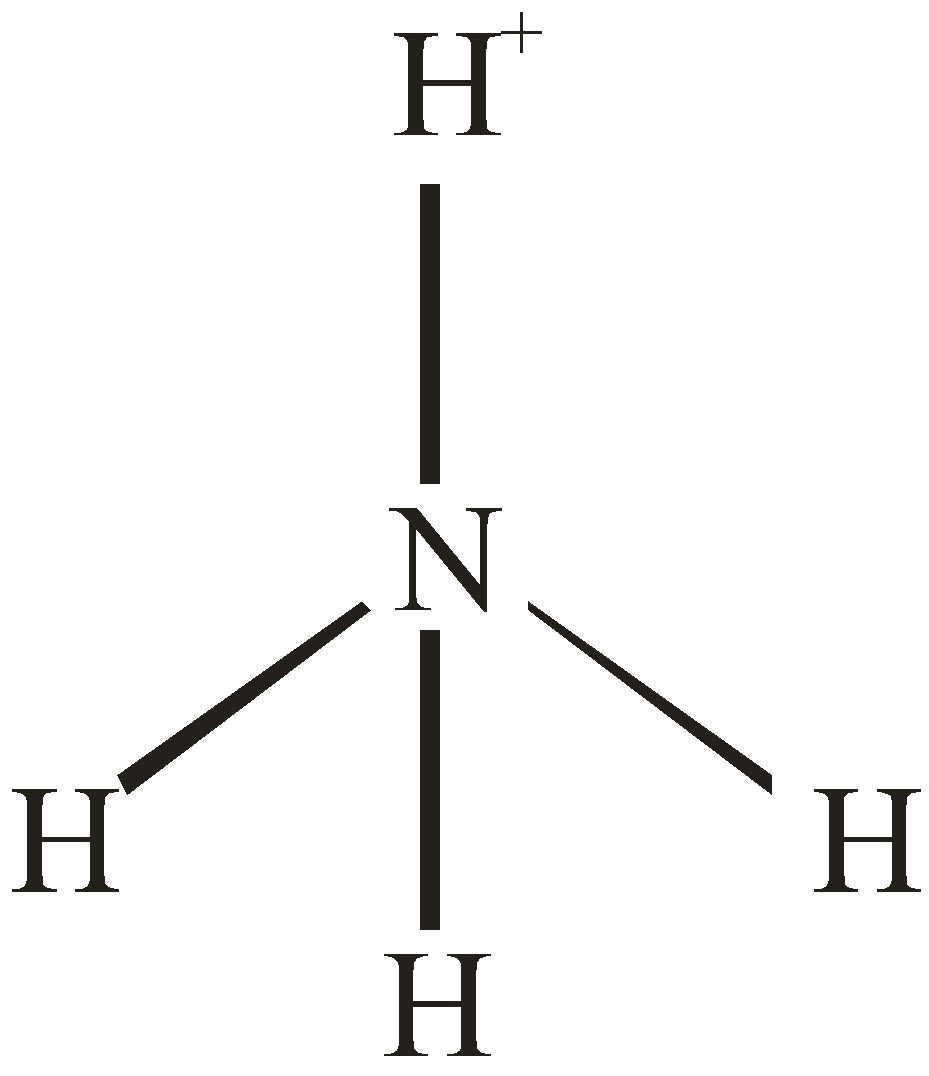
NH3 molecule NH4+ molecule
[Pyramidal structure] [Tetrahedral molecule]
(C) CO32− and CO2
Number of electron pair in CO32−=21[4+0+2]=3
The number of electron pairs are 3 that means the hybridisation will be sp2 and the geometry will be trigonal planar.
Number of electron pair in CO2=21[4+0+0]=2
Number of electron pairs are 2 that means the hybridisation will be sp and the geometry will be linear.
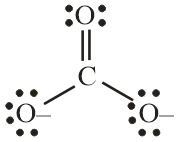
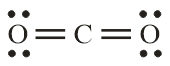
CO32− molecule CO2 molecule
(Trigonal planar structure) (Linear structure)
(D) CH4 and BF3
Number of electron pair in CH4=21[4+4+0]=4
Number of electron pair are 4 that means the hybridisation will be sp3 and the geometry will be tetrahedral
Number of electron pair in BF3=21[3+3+0]=3
Number of electron pairs are 3 that means the hybridisation will be sp3 and geometry will be trigonal planar.
Structures
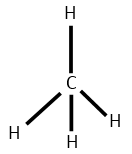
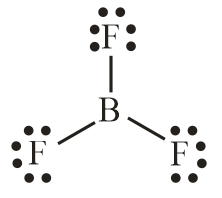
CH4 molecule BF3 molecule
(Tetrahedral structure) (Trigonal planar)
From this, we conclude that SO42− and BF4− have the same structure.
Hence, the correct answer is (A) SO42− and BF4−
Note: Geometry of a molecule is the arrangement of lone pair and bond pair while shape is the molecular structure excluding lone pairs of central atoms.
| S.No. | Molecules | Hybridization | Geometry | Molecular Structure |
|---|---|---|---|---|
| 1 | CH4 | sp3 | Tetrahedral | Tetrahedral |
| 2 | NH3 | sp3 | Tetrahedral | Pyramidal |
| 3 | H2O | sp3 | Tetrahedral | V-shape or Angular |
| 4 | BF3 | sp2 | Trigonal planar | Trigonal planar |
| 5 | BeH2 | sp | Linear | Linear |
