Question
Question: Which of the following pair of species have definite geometry?...
Which of the following pair of species have definite geometry?
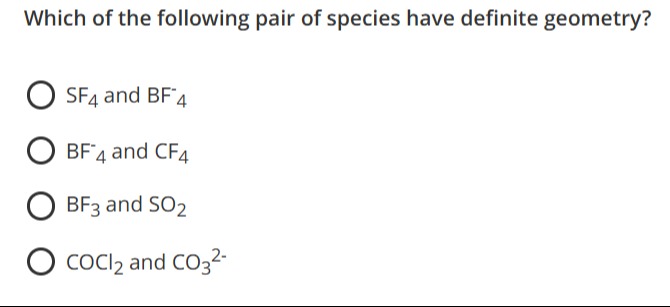
A
SF4 and BF4−
B
BF4− and CF4
C
BF3 and SO2
D
COCl2 and CO32−
Answer
BF4− and CF4
Explanation
Solution
All listed species have definite geometries. The question likely implies pairs with the same definite geometry or ideal definite geometry.
-
BF4−: Tetrahedral (ideal).
-
CF4: Tetrahedral (ideal).
- Pair (BF4− and CF4) has the same ideal geometry.
-
COCl2: Trigonal planar (ideal).
-
CO32−: Trigonal planar (ideal).
- Pair (COCl2 and CO32−) has the same ideal geometry.
Since both option 2 and option 4 consist of species with the same ideal geometry, and the question asks for "definite geometry", both pairs are valid. However, in a single-choice context, option 2 (BF4− and CF4) is often presented as a primary example of identical tetrahedral geometry.
