Question
Question: Which of the following oxyacids does not contain P-O-P bond? A. Isohypophosphoric acid B. Pyroph...
Which of the following oxyacids does not contain P-O-P bond?
A. Isohypophosphoric acid
B. Pyrophosphorous acid
C. Diphosphoric acid
D. Hypophosphoric acid.
Solution
The acid which contains oxygen is called oxyacid. If the oxygen is present in between two phosphorus atoms then the bond is called P-O-P bond. To find the P-O-P bond present in the given molecule the structure of the compound must be known.
Complete answer:
- In the question it is asked to find the compound which does not contain P-O-P bond in its structure among the given options.
- First of all, we should know the structure of the compounds which are present in the given options.
- Coming to option A, Isohypophosphoric acid.
- The structure of Isohypophosphoric acid is as follows.
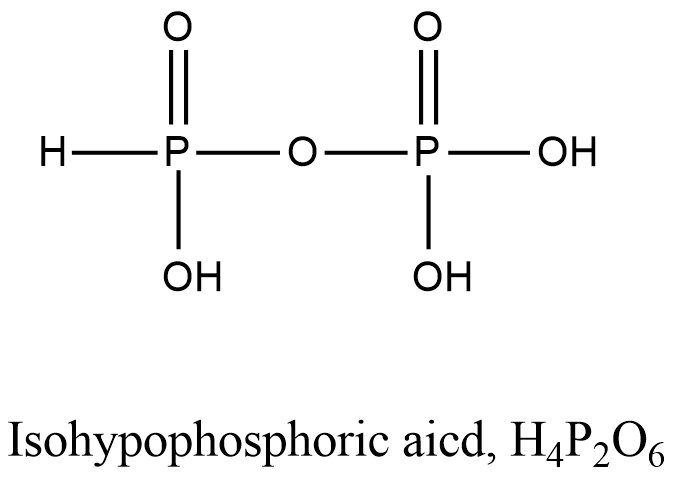
-In the structure of the Isohypophosphoric acid there is a presence of P-O-P bond.
- So, option A is incorrect.
- Coming to option B, Pyrophosphorous acid.
- The structure of Pyrophosphorous acid is as follows.
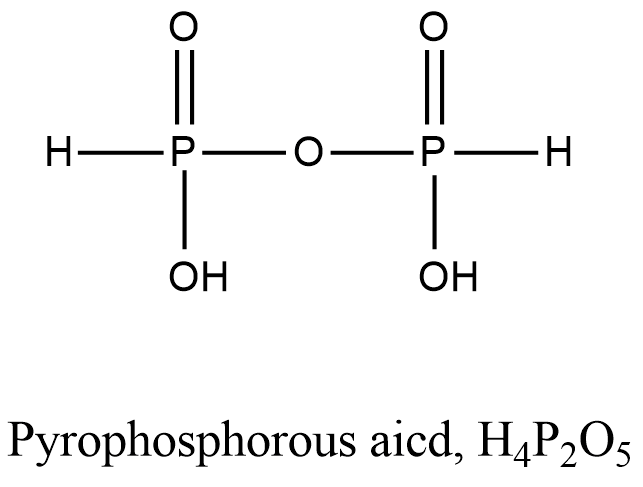
- In the structure of the Pyrophosphorous acid there is a presence of P-O-P bond.
- So, option B is incorrect.
- Coming to option C, Diphosphoric acid.
- The structure of Diphosphoric acid is as follows.
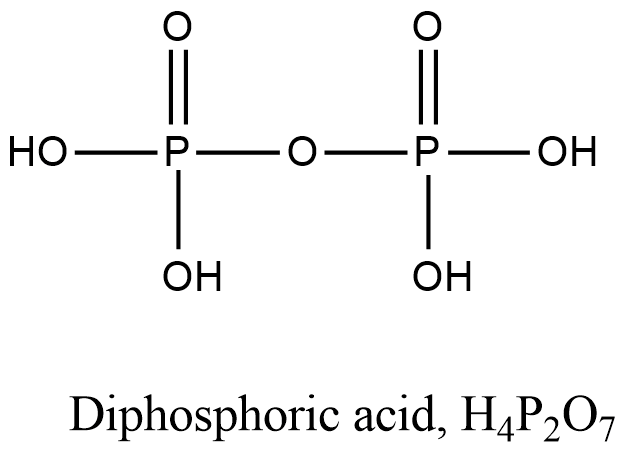
- In the structure of the Diphosphoric acid there is a presence of P-O-P bond.
- So, option C is incorrect.
- Coming to option D, Hypophosphoric acid.
- The structure of Hypophosphoric acid is as follows.
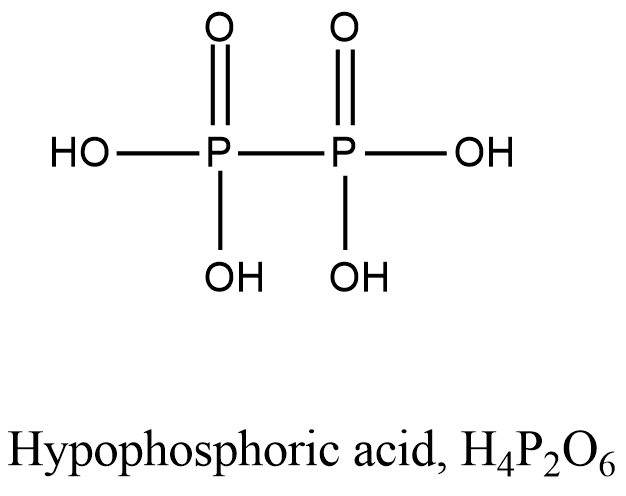
- In the above structure of the Hypophosphoric acid there is no P-O-P bond.
So, the option D is correct.
Note:
We have to draw the structures of the given compounds to know about the presence of the P-O-P bond. Without drawing the structures of the given compounds, we can find the presence of the P-O-P bond in the respective oxyacid.
