Question
Question: Which of the following oxides of nitrogen is a coloured gas? A. \({N_2}O\) B.NO C. \({N_2}{O_...
Which of the following oxides of nitrogen is a coloured gas?
A. N2O
B.NO
C. N2O4
D. NO2
Solution
Nitrogen dioxide appears as a reddish-brown gas. NO2 along with aerosols, is responsible for the reddish - brown colour of smog. Nitrogen dioxide partners to solid dinitrogen tetroxide at low temperatures.
Complete step by step answer:
Nitrous Oxide (N2O) or dinitrogen oxide is also known as Laughing Gas. Nitrous Oxide is a naturally occurring gas that is colourless and sweet-tasting gas. It is a non-flammable gas.
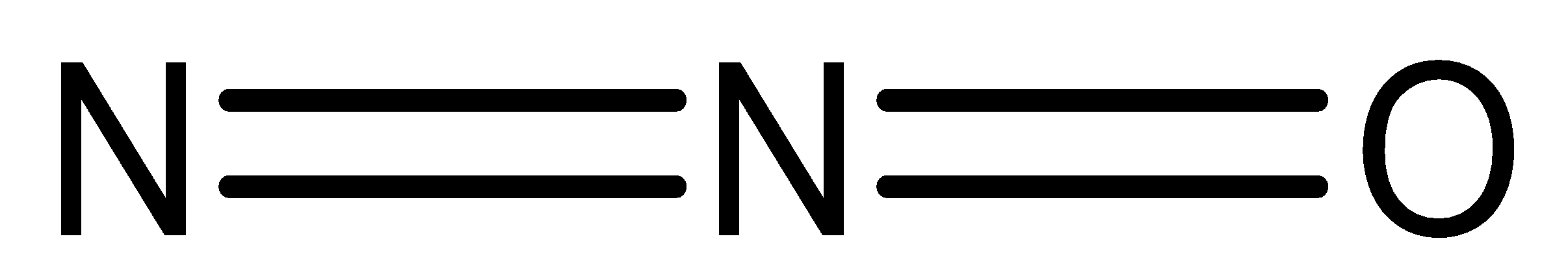
Nitric Oxide (NO) is a colourless gas. It achieves blue shade when it is melted. It is sparingly solvent in water and flammable gas with a slight odour. It is a molecule with a linear shape and is a resonance hybrid of the two structures. The bond length of NO is 115 pm which is intermediate between double bond and triple bond. And it is also paramagnetic in nature.
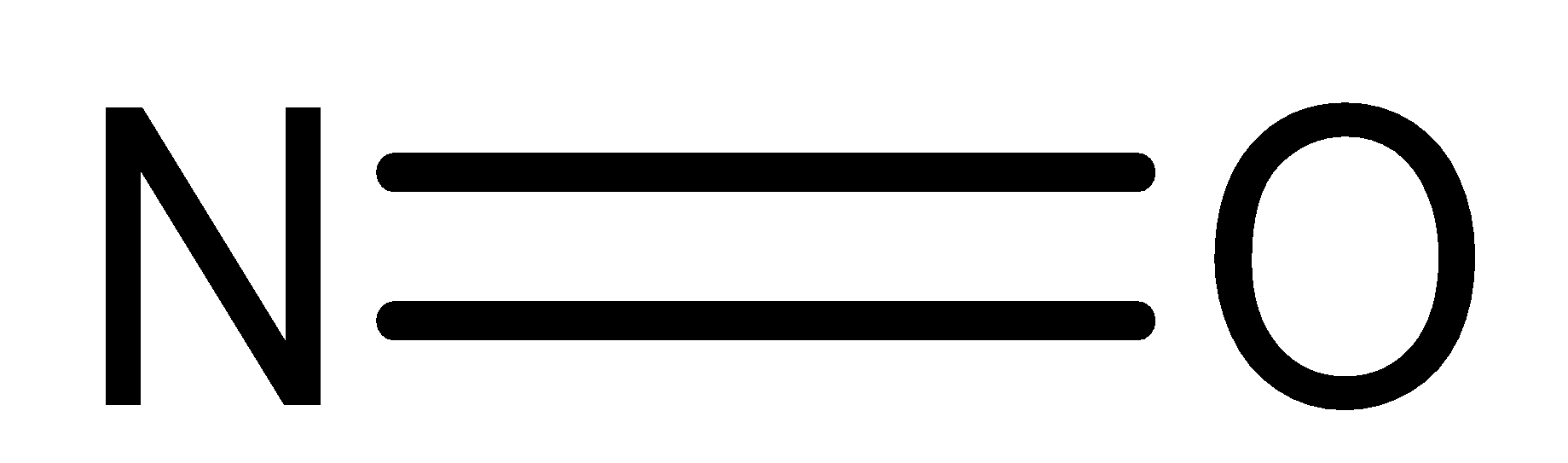
Dinitrogen tetroxide (N2O4) is a colourless gas. It has an irritating, unpleasant acid-like odour. N2O4 is a very reactive, toxic oxidizer. It is non-flammable with air but it will inflame combustible materials.
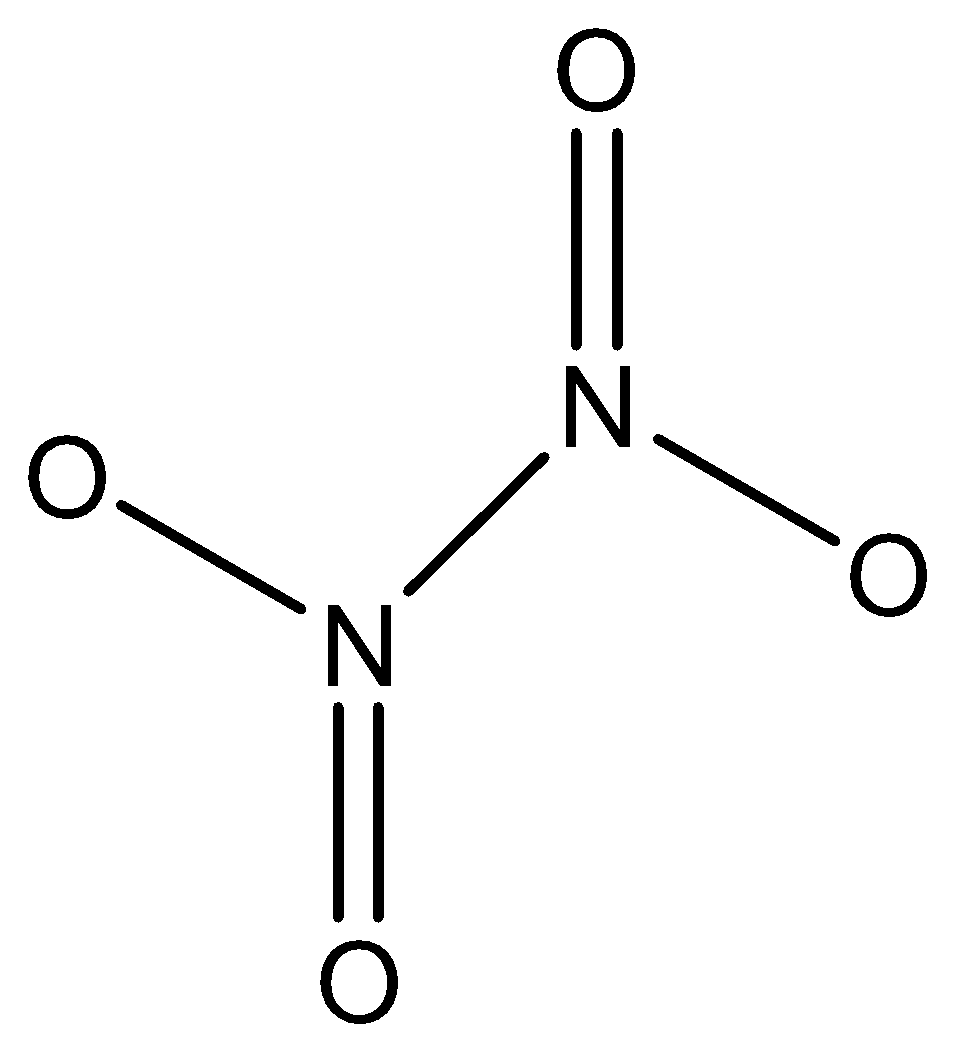
Nitrogen dioxide is a dark reddish-brown gas. It is poisonous but not flammable. NO2 along with aerosols, is responsible for the reddish - brown colour of smog. At high concentrations it is highly toxic, and can cause serious lung damage. Nitrogen dioxide is also a strong oxidising agent and thus it is very reactive with other compounds.
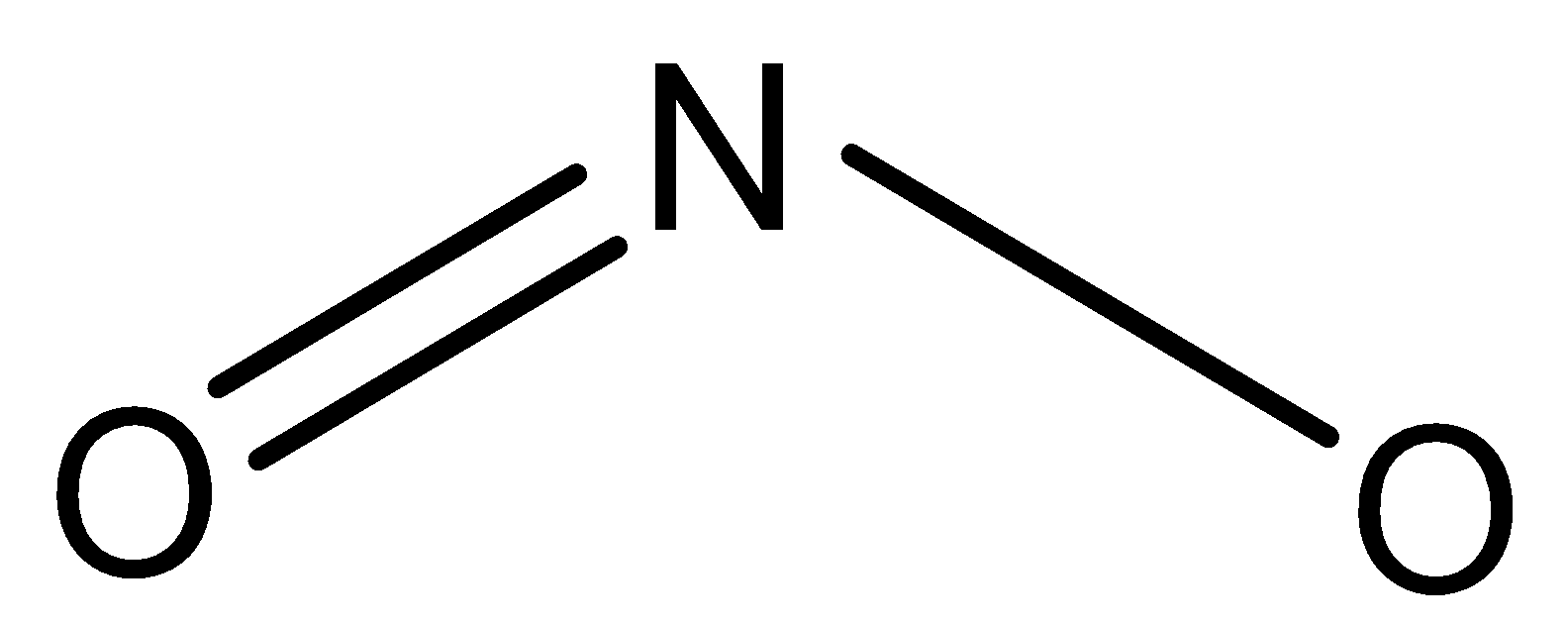
Therefore, the correct answer is option (D).
Note: Nitrogen dioxide is an acidic oxide with angular shape. Its bond angle is 134 degrees and bond length is 120 pm. The atom is found as a resonance hybrid and is a paramagnetic substance. Because of the nearness of unpaired electrons, it dimerizes to a colourless dinitrogen tetroxide (N2O4) atom that has an even number of electrons.
