Question
Question: Which of the following order(s) is/are correct regarding the property indicated?...
Which of the following order(s) is/are correct regarding the property indicated?
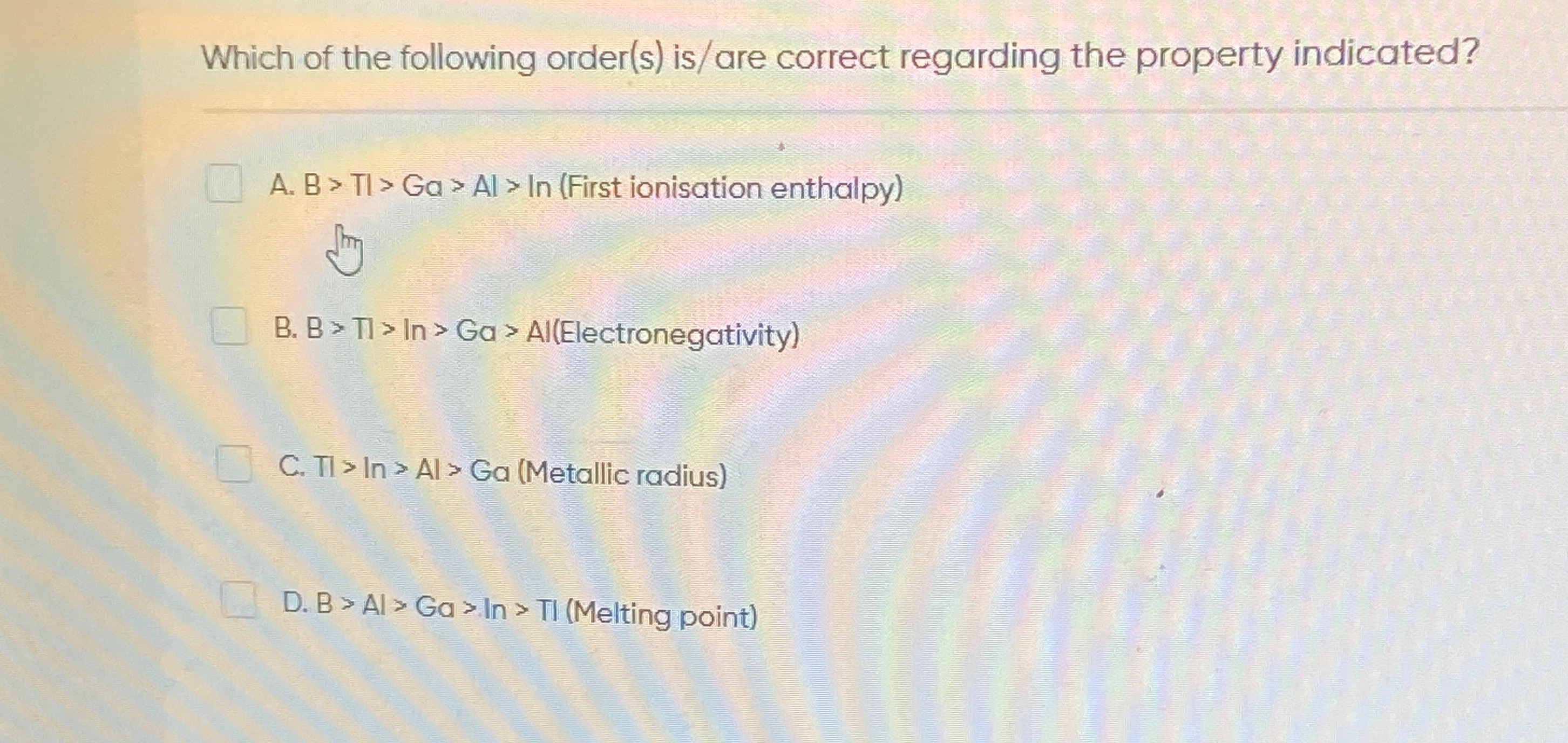
B > TI > Ga > Al > In (First ionisation enthalpy)
B > TI > In > Ga > Al(Electronegativity)
TI > In > Al > Ga (Metallic radius)
B > Al > Ga > In > TI (Melting point)
Options A and C.
Solution
Solution:
-
First Ionisation Enthalpy for B, Tl, Ga, Al, In:
Known values (in kJ/mol):
- B ≈ 800.6
- Tl ≈ 589.4
- Ga ≈ 578.8
- Al ≈ 577.5
- In ≈ 558.3
Order: B > Tl > Ga > Al > In.
Thus, Option A is correct.
-
Electronegativity for B, Tl, In, Ga, Al:
Pauling EN values (approx.):
- B ≈ 2.04
- Ga ≈ 1.81
- In ≈ 1.78
- Tl ≈ 1.62
- Al ≈ 1.61
Correct descending order should be: B > Ga > In > Tl ≈ Al.
Option B is not correct.
-
Metallic Radius for Tl, In, Al, Ga:
Metallic radius increases downward:
Approximate trend: Ga < Al < In < Tl
Thus order: Tl > In > Al > Ga.
Option C is correct.
-
Melting Point for B, Al, Ga, In, Tl:
Melting points (approx.):
- B ≈ 2349°C
- Al ≈ 660°C
- Tl ≈ 304°C
- In ≈ 156°C
- Ga ≈ 30°C
Correct descending order: B > Al > Tl > In > Ga.
Option D is not correct.
Core explanation:
Using known numerical data: Option A’s ordering for first ionisation enthalpy (B > Tl > Ga > Al > In) and Option C’s ordering for metallic radius (Tl > In > Al > Ga) match the trends. The other orderings don’t match the correct trends.
