Question
Question: Which of the following orders is correct for the no-bond-resonance energy of these radicals? 
(A) I>II>III
(B) III>II>I
(C) III>I>II
(D) II>III>I
Solution
Hint : In chemistry, radicals are also known as free radicals. Radicals are the molecules that must contain at least one unpaired electron and that electron is represented as a single dot or electron. The resonance energy represents the measure of the stability of the compound. It is generally calculated experimentally.
Complete Step By Step Answer:
In the question, we have the free radicals and we need to figure out the correct order of bond resonance energy. So, first, we will discuss the factors which affect the resonance energy of the molecule. One of the factors which affect the resonance energy is the canonical structures. More the number of canonical structures for the same molecule greater the stability of the compound. But this factor will not help us much here. So, we will use another factor that will increase the stability of compounds as well as the resonance bond energy. The factor is the number of hydrogen which can be represented as α−H . So, according to this factor greater, the number of α−H atoms greater will be the stability which will lead to greater bond resonance energy. So, now we will calculate the number of α−H an atom in each radical. α−H are the hydrogen which is bonded with the carbon atom attached next to the free radical carbon. So the calculated values are given below.
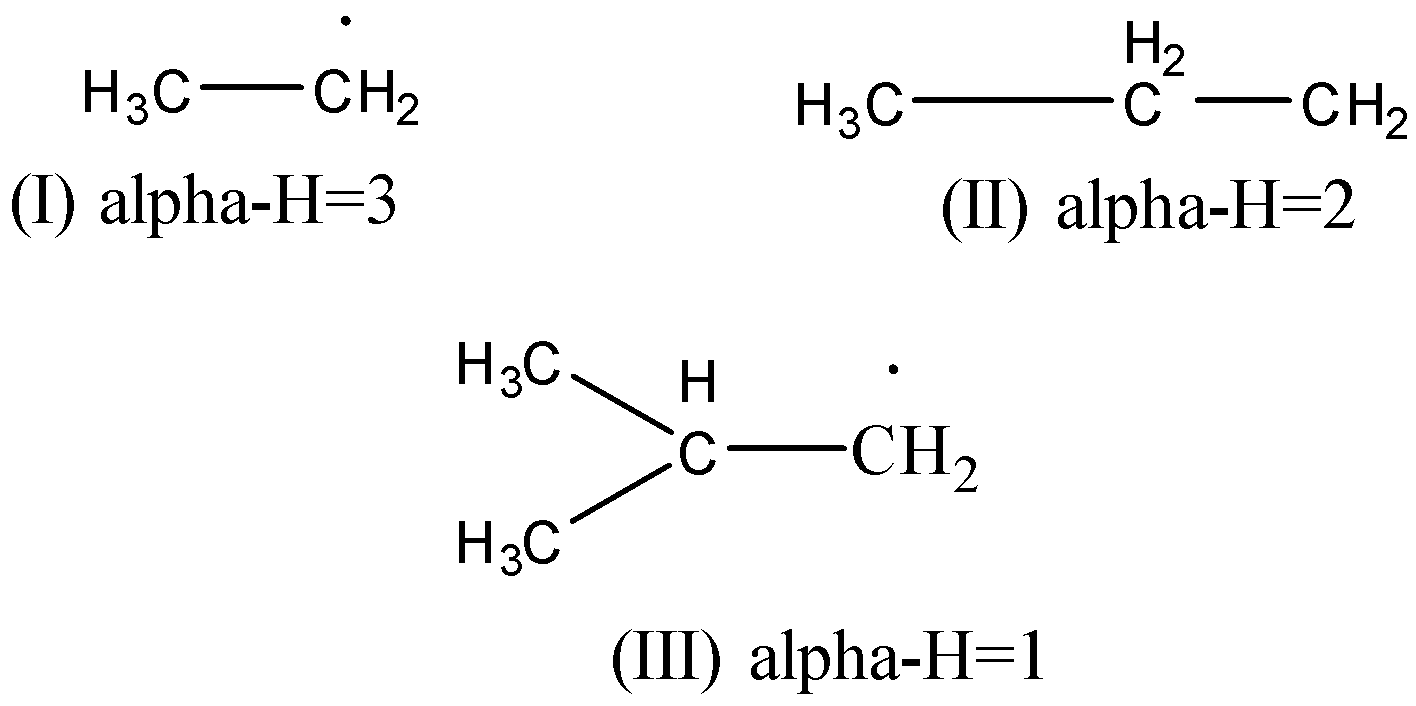
So, here we can observe the number of α−H the atom and conclude the order as I>II>III .
Therefore, the correct option is (A).
Note :
No bond resonance is also known as “Baker-Nathan-effect”. If we calculate the value of resonance energy of any compound it will always be negative. It measures the energy difference between the real structures and the most stable resonating structure of the molecule.
