Question
Question: Which of the following order is/are INCORRECT with respect to the given property? NaF < NaCl < NaBr...
Which of the following order is/are INCORRECT with respect to the given property?
NaF < NaCl < NaBr < NaI : Ionic character
SrC2O4 < BaC2O4 < BeC2O4 : Solubility in water
Ca3N2 < Mg3N2 < Be3N2 : Thermal stability
AgI < AgBr : covalent character
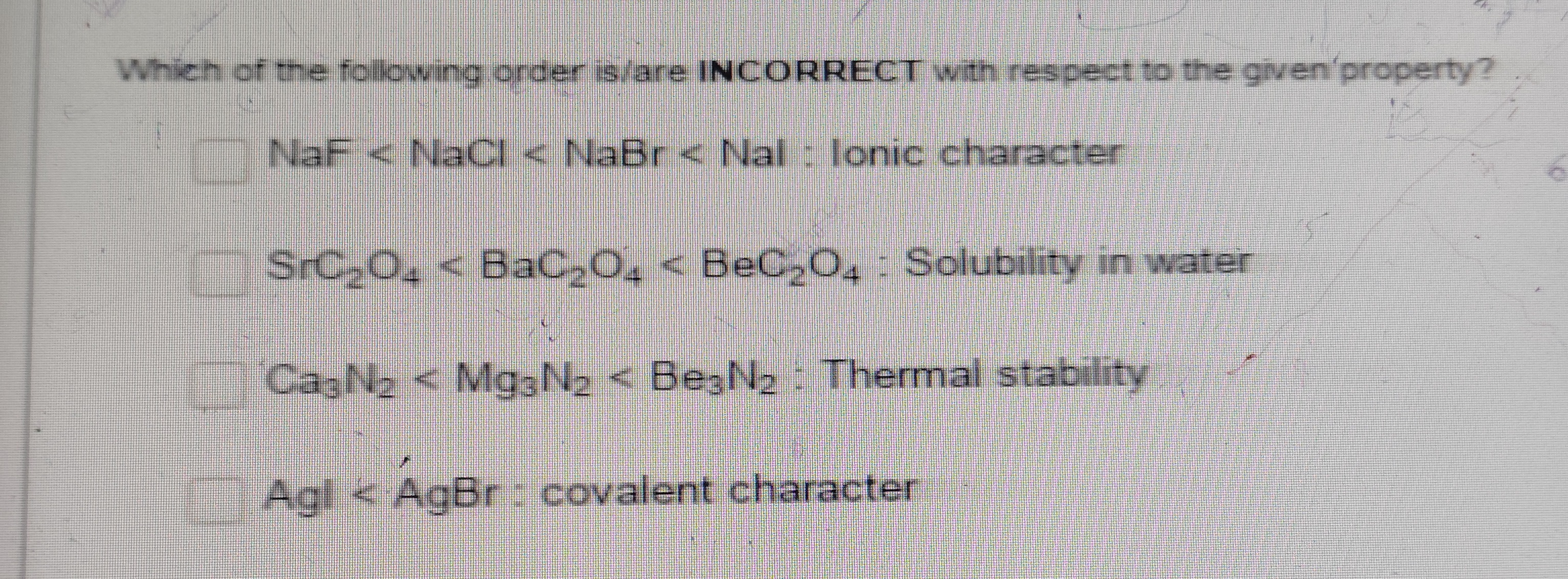
NaF < NaCl < NaBr < NaI : Ionic character
SrC2O4 < BaC2O4 < BeC2O4 : Solubility in water
Ca3N2 < Mg3N2 < Be3N2 : Thermal stability
AgI < AgBr : covalent character
NaF < NaCl < NaBr < NaI : Ionic character, SrC2O4 < BaC2O4 < BeC2O4 : Solubility in water, AgI < AgBr : covalent character
Solution
The question asks to identify the incorrect order(s) among the given statements. Let's analyze each statement:
-
NaF < NaCl < NaBr < NaI : Ionic character
The ionic character of a bond is related to the electronegativity difference between the bonded atoms. For alkali metal halides (NaX), the cation is Na+, and the anions are F−, Cl−, Br−, and I−. The electronegativity of halogens decreases down the group: F > Cl > Br > I. Thus, the electronegativity difference between Na and the halogen decreases from F to I: ΔEN(Na-F)>ΔEN(Na-Cl)>ΔEN(Na-Br)>ΔEN(Na-I). A larger electronegativity difference implies greater ionic character.
Therefore, the correct order of ionic character is NaF > NaCl > NaBr > NaI.
The given order NaF < NaCl < NaBr < NaI represents increasing ionic character, which is incorrect.
-
SrC2O4 < BaC2O4 < BeC2O4 : Solubility in water
These are oxalates of alkaline earth metals. The anion (C2O42−) is large. For salts with large anions, the solubility in water generally decreases down the group of alkaline earth metals. This trend is primarily influenced by the hydration energy of the cations, which decreases significantly down the group (Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+) as the ionic size increases and charge density decreases. Although lattice energy also decreases down the group, the decrease in hydration energy is typically more dominant for salts with large anions, leading to a decrease in solubility.
The correct order of solubility is BeC2O4 > MgC2O4 > CaC2O4 > SrC2O4 > BaC2O4.
Arranging the given oxalates (Sr, Ba, Be) in order of increasing solubility according to the correct trend: BaC2O4 < SrC2O4 < BeC2O4.
The given order is SrC2O4 < BaC2O4 < BeC2O4. Comparing this with the correct order, the relative position of Sr and Ba is reversed (Sr is more soluble than Ba). Therefore, the given order is incorrect.
-
Ca3N2 < Mg3N2 < Be3N2 : Thermal stability
The thermal stability of ionic compounds is generally related to their lattice energy. Higher lattice energy leads to greater thermal stability. For alkaline earth metal nitrides (M3N2), the lattice energy is proportional to r1+r2Z1Z2, where Z1 and Z2 are the charges of the ions and r1 and r2 are their radii. The charges (+2 for M2+, -3 for N3−) are constant. The ionic radii of the cations increase down the group: Be2+ < Mg2+ < Ca2+. The radius of the anion (N3−) is constant. Thus, the internuclear distance (r1+r2) increases from Be3N2 to Ca3N2.
Lattice energy decreases as the internuclear distance increases: LE(Be3N2) > LE(Mg3N2) > LE(Ca3N2).
Therefore, the thermal stability order is Be3N2 > Mg3N2 > Ca3N2.
The given order Ca3N2 < Mg3N2 < Be3N2 represents increasing thermal stability, which is consistent with the correct trend. This order is correct.
-
AgI < AgBr : covalent character
The covalent character in ionic compounds can be explained using Fajan's rules. According to Fajan's rules, covalent character increases with increasing polarizability of the anion. For silver halides (AgX), the cation is Ag+, and the anions are Br− and I−. The polarizability of halide ions increases down the group: F− < Cl− < Br− < I−.
Therefore, the covalent character of silver halides increases in the order AgF < AgCl < AgBr < AgI.
The correct order of covalent character between AgBr and AgI is AgBr < AgI.
The given order AgI < AgBr represents AgI having less covalent character than AgBr, which is incorrect.
Based on the analysis, the incorrect orders are statements 1, 2, and 4.
