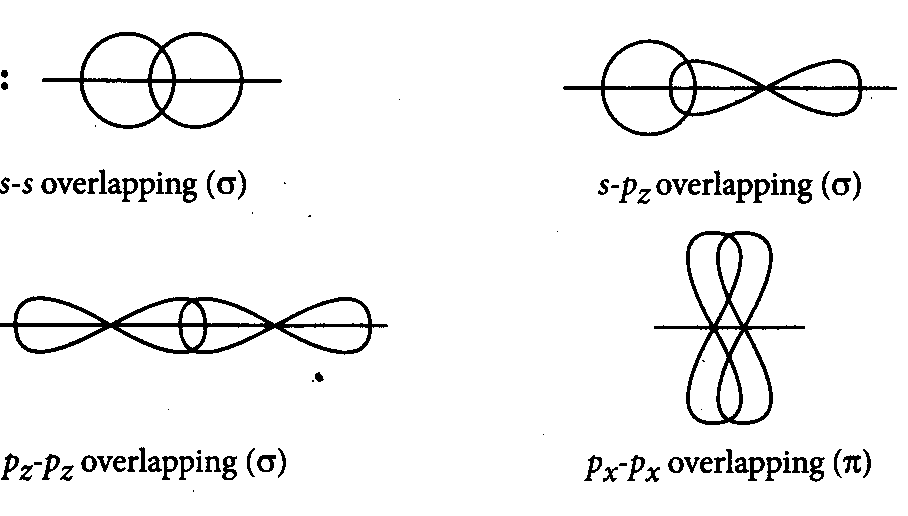
Question
Question: Which of the following orbitals will not form sigma bond after overlapping ?...
Which of the following orbitals will not form sigma bond after overlapping ?
A
s-orbital and s-orbital
B
s-orbital and pz- orbital
C
pz−orbital and Pzorbital
D
Px−orbital and Px−orbital
Answer
Px−orbital and Px−orbital
Explanation
Solution
: