Question
Question: Which of the following orbitals are degenerate?...
Which of the following orbitals are degenerate?
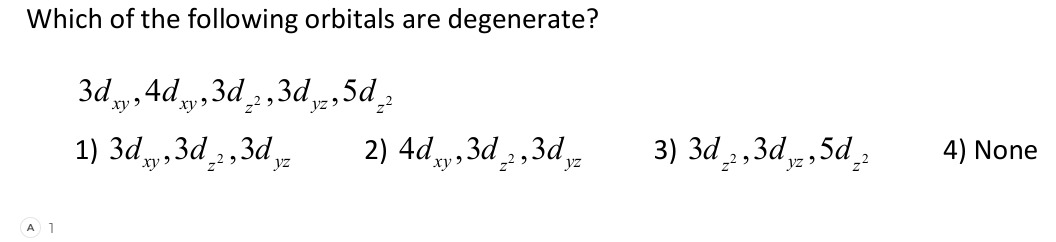
A
3dxy,3dz2,3dyz
B
4dxy,3dz2,3dyz
C
3dz2,3dyz,5dz2
D
None
Answer
3dxy,3dz2,3dyz
Explanation
Solution
Degenerate orbitals have the same principal quantum number n and same angular quantum number l.
All the orbitals in option 1 are 3d orbitals (n=3, l=2), hence they are degenerate.
Options 2 and 3 mix different n values (3 and 4 or 3 and 5), so they are not degenerate.
