Question
Question: which of the following names does not fit like a real name? A.3-methyl 3-hexanone B.4-methyl 3-h...
which of the following names does not fit like a real name?
A.3-methyl 3-hexanone
B.4-methyl 3-hexanone
C.3-methyl 3-hexanol
D.2-methylcyclohexanone
Solution
The IUPAC nomenclature of the organic compounds is done on the basis of a fixed set of rules and the substituents attached to the carbon atoms are named based on their preference. Each carbon forms four bonds exactly.
Complete step by step answer:
3-methyl 3-hexanone should look like:
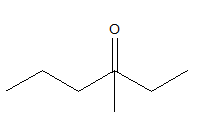
According to the name, as there is “hex”, the compound should be a six membered chain. At the third carbon of the chain there should be a carbonyl bond. But that satisfies the four valences of carbon and it cannot form another bond with a methyl group.
4-methyl 3-hexanone
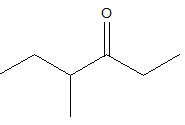
The structure of the compound should be like this as above. The carbonyl group is at the third position and the methyl group is at the fourth position.
3-methyl 3-hexanol
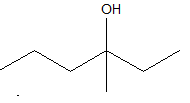
As, the hydroxyl group has a single bond with the carbon atom, so it can form one more bond with the methyl group at the third position. Hence 3-methyl 3-hexanol is possible.
2-methylcyclohexanone
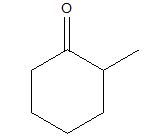
In 2-methylcyclohexanone, there should be a cyclohexane ring, to which at the axial position or the first position there should be a carbonyl group and at the second position, there should be a methyl group. So the structure lives up to its name and hence the name is justified.
So seeing the structure it can be said that only the first name cannot have a structure due to the valency satisfaction of the third carbon by the carbonyl group.
Hence, the correct option is A.
Note:
According to the rules of organic nomenclature, the longest chain of carbon is considered and the naming should start from the end closest to the substituents attached to the carbon atom of the main chain. The substituents have an order of preference and the highest priority group is named at the end of the compound. For example, if a ketone group is present then “one” comes at the end of the compound.
