Question
Question: Which of the following molecules(s) is/are having pyramidal structure? (a)\(ClO_{3}^{-}\) (b)\({...
Which of the following molecules(s) is/are having pyramidal structure?
(a)ClO3−
(b)H3O+
(c)NH3
(d)PCl3
Solution
The question contains multiple correct options. The shape of the molecule can be known by checking its hybridization and the number of lone pairs and bond pairs present in the molecule. The hybridization is calculated using the formulae,
Z=21(no.ofvalencee-sofcentralatom+no.ofmonovalentatomsattached+negativechargeifany-positivechargeifany)
Complete answer:
So in the question, it is asked to comment that from the given options which molecules will have a pyramidal shape and a hint is also given that there is more than one correct answer for this question.
To find the shape or geometry of the compounds given, first we have to know its hybridization.
If the hybridization is known then we could say the shape of the molecule by considering the number of lone pairs and bond pairs present.
So we can find the hybridization of a compound using the formulae,
Z=21(no.ofvalencee-sofcentralatom+no.ofmonovalentatomsattached+negativechargeifany-positivechargeifany)
Let us consider the first option, ClO3−.Here Cl is the central atom and it has 7 valence electrons and three oxygen atoms attached which is a divalent atom and a negative charge.
So if we calculate the hybridization of the compound using the formulae we get,
Z=21(7+1)=28=4
So we got the valueZ=4, hence the hybridization associated with the molecule is sp3.
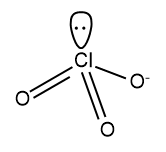
Four sp3 orbitals will be formed and three will be utilized to bond with O and one will be unoccupied and will remain as the lone pair. The tetrahedral geometry associated withsp3 hybridization will be distorted to pyramidal geometry due to the presence of the lone pair.
The structure of ClO3− will be:
Now let us consider option (b), H3O+
Here O is the central metal atom with 6 valence electrons and three H attached to them and the O having a positive charge,
We get the value of Z as, Z=21(6+3−1)=28=4
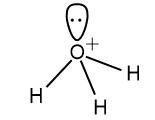
Here also the value of Z is 4 and the hybridization will be sp3 hybridization. Four sp3 hybridized orbitals will be formed in which three are utilized to bond with H and the other remains as unoccupied i.e. as lone pairs. Hence the tetrahedral structure will be distorted to pyramidal structure.
The structure of H3O+ will be:
Now let’s consider option (c), which is NH3.Here the central atom is N with 5 valence electrons and with 3 H atoms attached to them.
The value of Z will be, Z=21(5+3)=28=4
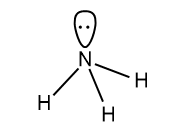
The hybridization of the molecule will be sp3 hybridization. From the four sp3hybrid orbitals formed, three will be combined with H and the fourth one will remain as lone pair due to which the geometry of the compound is distorted to pyramidal structure.
The structure of ammonia will be:
Now let’s move to the last option, PCl3
The case of PCl3 is very much similar to NH3, since both the central atoms belong to the same group. P is the central atom with five valence electrons and three monovalent Cl atoms attached to the central atom.
The value of z will be, Z=21(5+3)=28=4
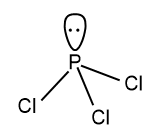
The hybridization will be sp3 hybridization. Here four sp3 orbitals are formed and three are used to bond with the Cl atom and the one remains as unoccupied as a lone pair. Due to the presence of the lone pair the shape of the compound is distorted to pyramidal structure.
The structure of the compound will be:
Hence all the four options given have pyramidal structure.
Note:
The hybridization changes with the value of Z obtained.
If the value of: Z=2,sphybridised
If Z=3,sp2hybridised
If Z=4,sp3hybridised
If Z=5,sp3dhybridisedetc.
The geometry of the compound depends on the hybridization, but its actual shape depends on the number of one pairs and bond pairs present and in the arrangement of the lone pairs and bond pairs in space.
