Question
Question: Which of the following molecules is planar? (A) \(N{F_3}\) (B) \(NC{l_3}\) (C) \(P{H_3}\) (D...
Which of the following molecules is planar?
(A) NF3
(B) NCl3
(C) PH3
(D) BF3
Solution
Geometry of molecules depends on hybridization. Hybridization is a process of mixing and reacting of atomic orbitals of the same energy and form the same number of hybrid orbitals of the same energy.
Complete step by step answer:
Let us discuss hybridization in the following molecules NF3,NCl3,PH3,BF3one by one.
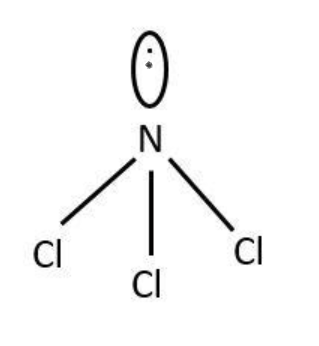
Hybridization in NCl3-
Central atom of these molecules is Nitrogen.
Electronic configuration of N=1s22s22px12py12p21
At ground state
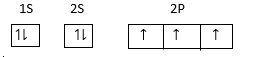
Electronic configuration of N in excited state =
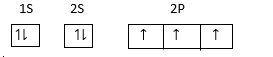
Electrons from 2s orbitals jump to 2p orbitals excitation takes place at the same energy level.
Now one 2s and two 2p orbitals hybridize and form Four sp3hybrid orbitals. Therefore, hybridization is tetrahedral with one lone pair of electrons.
Electronic Configuration of Cl is: 1s22s22p63s23px13py13p21
Thus, three unpaired electrons of hybrid orbitals of N form a covalent bond with unpaired electrons of 3p2 orbital.
Due to the presence of unpaired electrons, the geometry is distorted tetrahedral and not planes.
Hybridization in NF3:
Following the same steps in NF3 as NCl3.
We will give electronic configuration of ‘F’ in place of ‘Cl’.
Again hybridization is distorted tetrahedral due to the presence of lone pairs of electrons.
Hybridization in PH3: Central atom is P.
Electronic configuration of P is =1s22s22p63s23px13py13p21
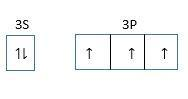
One s and three p orbitals hybridize and form Four sp3 hybrid orbitals of which one orbital has lone pair and three have unpaired electrons.
Three hydrogen atoms form three covalent bonds with unpaired electrons of hybrid orbital. Hybridization is sp3.
The geometry is distorted tetrahedral due to the presence of lone pairs of electrons.
Hybridization in BF3: Central atom is B.
Electronic configuration is =1s22s22p1
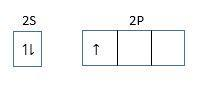
Electronic configuration in an excited state.
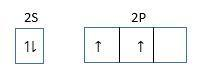
One 2s and two 2p orbital hybridize and form three sp2 hybridized orbitals. There are no lone pairs of electrons in hybrid orbital so geometry is planar and it is trigonal planar.
Fluorine has Electronic configuration, 1s22s22px22py22p21
2p2 electron forms covalent bond with unpaired electron of B.
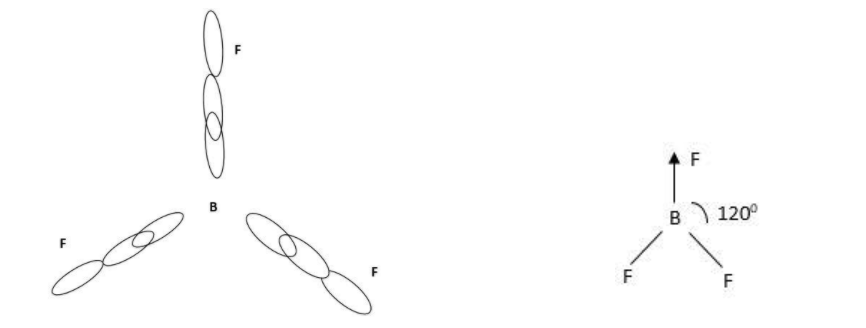
Geometry is trigonal planar and angle between them is 1200.
So, the correct answer is “Option D”.
Note:
The repulsion between lone pair and bond pair of electrons is more. Therefore, distortion takes place. But there is no repulsion between bond pair and bond pair of electrons.
Therefore geometry in BF3 is planer.
