Question
Question: Which of the following molecules is of T-shape? (A) \( {{I}_{3}}. \) (B) \( Cl{{F}_{3}}. \) ...
Which of the following molecules is of T-shape?
(A) I3.
(B) ClF3.
(C) SF4
(D) XeF4
Solution
We know that the valence shell electron pair repulsion (VSEPR) theory is used to determine the shape of molecules. According to VSEPR theory the lone pair-lone pair has the maximum repulsion followed by the bond pair-lone pair and bond pair –bond pair. In ClF3 two lone pairs are placed in an axial plane which minimizes the repulsion and stabilizes the molecule.
Complete answer:
In, the chlorine is a central atom. The electronic configuration of chlorine atom is as: Cl=1s22s22p63s23p5.
Chlorine atom has seven valence electrons. In molecules, the central chlorine atom is surrounded by three fluorine atoms. Each fluorine atom shares one electron with chlorine atom and results in three covalent Cl−F bonds. The ClF3 molecule has 7 valence electrons from chlorine and three from Fluorine. Thus there are a total 10 electrons around the central chlorine atom. Now let’s determine the electron pair in ClF3 molecule. Divide the total number of electrons by 2.
There are a total of five electron pairs in molecules. Let’s calculate the number of lone pairs in the molecule. The lone pair of electrons can be determined by subtraction of the number of bond pairs from the electron pair.
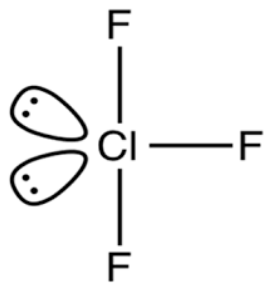
Therefore, the correct answer is option B.
Note:
Remember that while drawing the structures for different molecule types; always highlight the lone pairs to easily calculate the number of lone pairs. Remember that the shape and geometry of a molecule are not always the same. When lone pairs are present, the shape of the molecule is different from the geometry of the molecule.
