Question
Question: Which of the following molecules is not hypovalent? A. \({\text{AlC}}{{\text{l}}_{\text{3}}}\) ...
Which of the following molecules is not hypovalent?
A. AlCl3
B. AlBr3
C. AlF3
D. All are hypovalent Because AlF3 is an ionic compound
Solution
The molecules in which the central atom has less than 8 valence electrons are known as hypovalent molecules. Central atom hypovalent molecules do not obey the octet rule. The number of electrons in the valence shell of the central atom also depends on the type of bonding.
Complete step by step answer:
Determine the type of bond present in given compounds as follows:
In given compounds, central atom (Al) is common only there is a change in bonding atom. Atoms F,Cland Br are all halogens and present in the same group of the periodic table.
Atomic size of elements increases as we move from top to bottom in the periodic table. So the increase in order of the atomic size of F,Cland Br is as follows :
F < Cl < Br
Similarly, the order of the ionic size of F - ,Cl - and Br - is as follows :
F - < Cl - < Br -
The electronegativity of elements decreases as we move from top to bottom in the periodic table. So the decrease in order of electronegativity of F,Cland Br is as follows:
F > Cl > Br
In the case of compounds AlF3,AlCl3and AlBr3 cation (Al3 + ) is common so an increase in the size of anion increases the covalent character of compounds. Due to the greater size of anions Cl - and Br - compounds AlCl3and AlBr3 are covalent compounds while due to greater electronegativity and smaller size of F - ion compoundAlF3 is an ionic compound.
Using the type of bond present determines the number of electrons in the valence shell of the central atom for all given compounds.
Atomic number of Al is 13
So, the electronic configuration of a neutral Al atom is 2, 8, 3
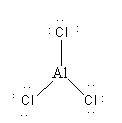
AlCl3 is a covalent compound so during covalent bond formation central Al atom shares its 3 valence electrons with three Cl atoms. As the central atom has less than 8 electrons so AlCl3 is hypovalent compound.
So, option (A) AlCl3 is not the correct option.
AlBr3 is a covalent compound so during covalent bond formation central Al atom shares its 3 valence electrons with three Br atoms. As the central atom has less than 8 electrons so AlBr3 is hypovalent compound.
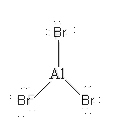
So, option (B) AlBr3 is not the correct option.
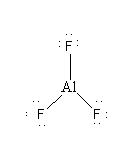
AlF3 is an ionic compound so during ionic bond formation central Al atom donates its outermost 3 electrons. So, Al3 + ion in AlF3 has electronic configuration 2, 8. As the octet of the central atom is fulfilled so AlF3 is not hypovalent compound.
Thus, the correct option is C.
Note: Though in all compoundsAlF3,AlCl3and AlBr3there is a bond between the metal and non-metal only AlF3 is ionic compound while AlCl3and AlBr3 are covalent compounds
