Question
Question: Which of the following molecules has the maximum dipole moment? (A) \(C{O_2}\) (B) \(C{H_4}\) ...
Which of the following molecules has the maximum dipole moment?
(A) CO2
(B) CH4
(C) NH3
(D) NF3
Solution
Dipole moment can be defined as the product of the distance between the two atoms and the charge on the atoms. The atoms forming bonds need to have differences in electronegativity in order to generate dipole moment.
Complete answer:
When a covalent bond is formed between the same atoms, the pair of electrons is equally attracted by both of the atoms. When a bond is formed between different atoms, the pair of electrons is not equally attracted by both of the atoms. This leads to polarization.
- Due to this polarization the bond possesses a dipole moment. This dipole moment can be defined as the product of the distance between the two atoms and the charge on the atoms.
- CO2 :
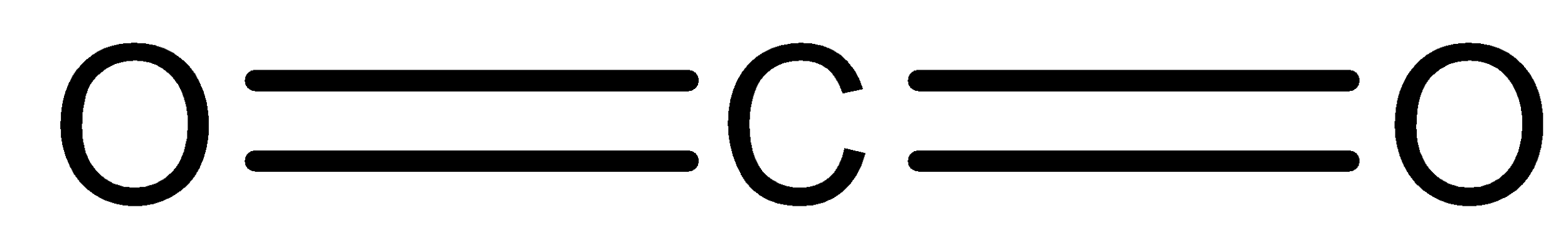
We can see that it is a linear molecule. It involves two C-O double bonds. So, as the electronegativity of carbon atom and oxygen atom is different, there will be some moment in both the bonds. But the direction of the moment will be exactly opposite to each other. So, they will cancel each other. Thus, we can say that it does not have any dipole moment.
- CH4 :
This molecule also has a tetrahedral shape. There is a moment present in C-H bond but all the moments will be cancelled as they all nullify their moment upon combination.
- NH3 :
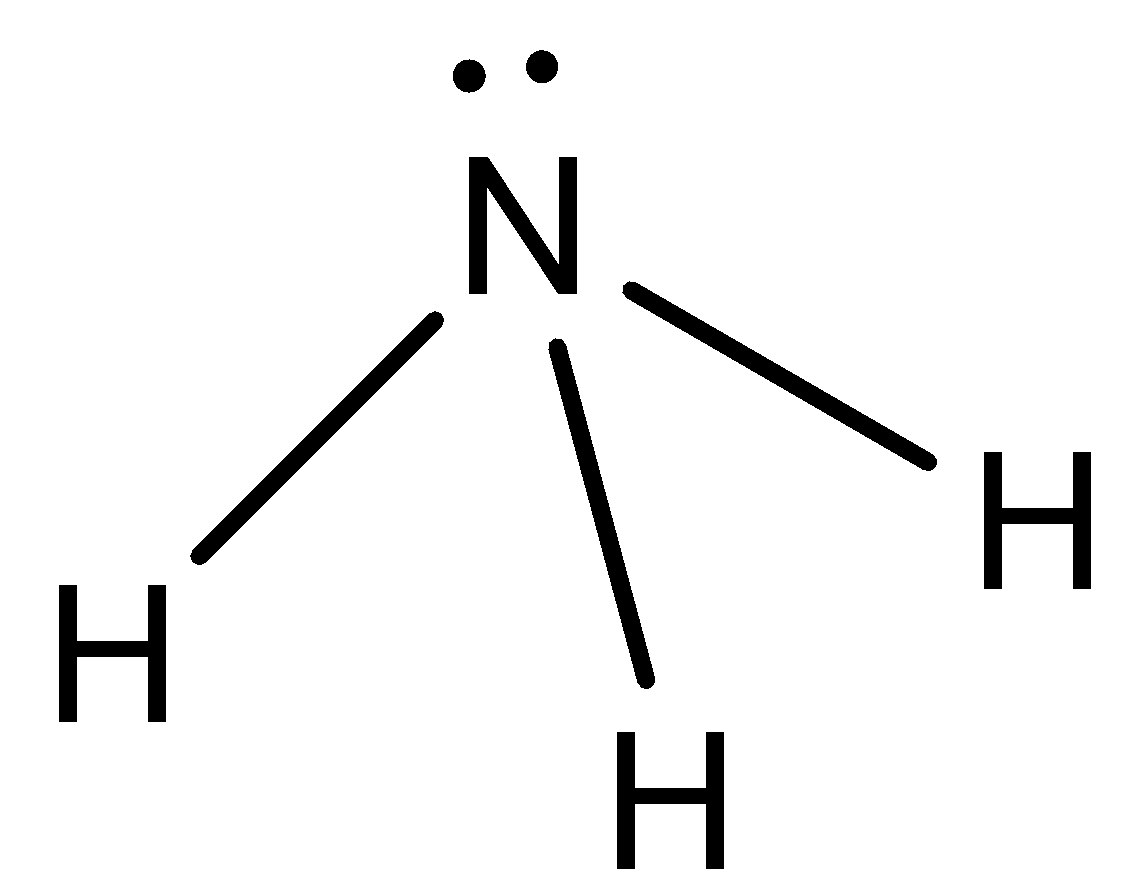
Here, we can see that the difference in electronegativity between N and H atoms is huge. So, there will be some dipole moment in the molecule.
- NF3:
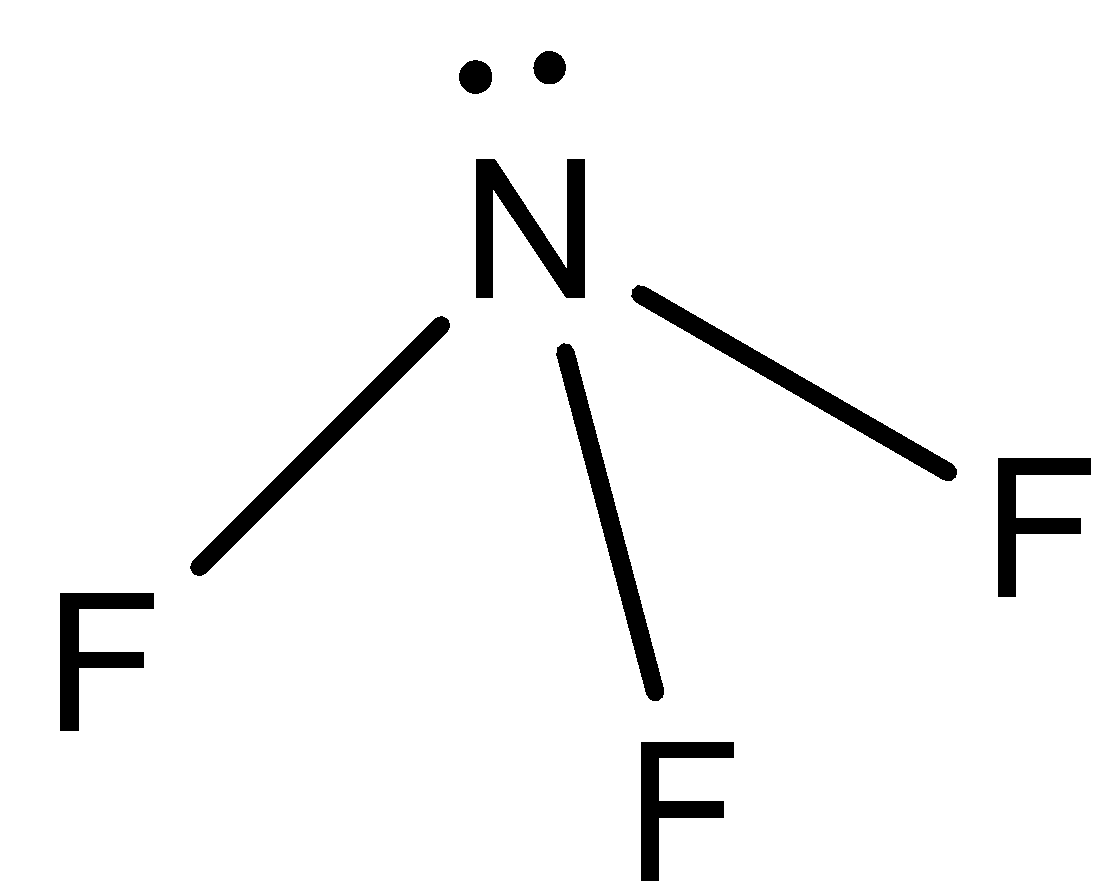
This molecule has the same shape as ammonia but it has fluorine atoms in place of hydrogen atoms. The difference in electronegativity between nitrogen and fluorine atoms is less as compared to N and H atoms. So, there will be less dipole moment in NF3 as compared to ammonia.
Thus, we can conclude that the correct answer is (C).
Note:
The dipole moment of a bond is expressed in the Debye unit. It is designated by μ. The resultant dipole moment of NH3 is 4.90×10−30Cm and that of NF3 is 0.80×10−30Cm.
