Question
Question: Which of the following molecules has the highest dipole moment? A. \({H_2}S\) B. \(C{O_2}\) ...
Which of the following molecules has the highest dipole moment?
A. H2S
B. CO2
C. CCl4
D. BF3
Solution
Dipole moment occurs only when there is a separation of charge. Dipole moments can occur between two ions in a compound with an ionic bond or between two atoms with a covalent bond. Dipole moment depends upon the distance between the charges. It also depends upon the structure of the compound.
Complete step by step answer:
We are given four compounds and we have to determine the compound with the highest dipole moment.
CO2 is called carbon dioxide. In CO2 molecule, two oxygen atoms form a bond with the carbon atom. There are two carbon-oxygen double bonds in CO2, O=C=O . The structure of CO2 is linear and symmetrical. Therefore, the dipole moment of one carbon-oxygen double bond gets canceled with the dipole moment of another carbon-oxygen double bond because of the opposite directions. So the net dipole moment is zero.
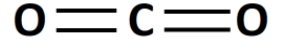
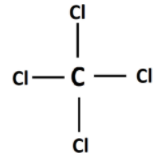
CCl4 is carbon tetrachloride. In CCl4, the chlorine atoms are positioned around the carbon atom. The chlorine atoms are put in the corners in tetrahedral configuration symmetrically. The net dipole moment thus becomes zero.
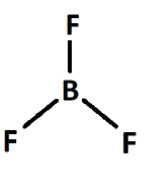
In BF3, the fluorine atoms are positioned around boron with boron-fluorine single bonds. The angle between two consecutive bonds is 120 degrees. So the net dipole moment of the three bonds becomes zero.
Therefore, H2S has the highest dipole moment.
The correct option is Option A.
Note:
The bond angle in a linear molecule is 180 degrees, in a symmetrical tetrahedral molecule it is 90 degrees and in planar molecules, it is 120 degrees. The dipole moment is a vector quantity, therefore it has a direction. So in a linear molecule if one dipole moment is positive then the other will be negative thus the net dipole moment is zero.
