Question
Question: Which of the following molecules has complete octet? A) \(BeC{l_2}\) (dimer) B) \(Be{H_2}\) (dim...
Which of the following molecules has complete octet?
A) BeCl2 (dimer)
B) BeH2 (dimer)
C) BeH2 (s)
D) BeCl2 (s)
Solution
We have to understand that a molecule attains complete octet when the central atom is surrounded by eight electrons i.e. when its valence shell contains noble gas configuration. This can be checked by finding the valency of the atoms present and the electrons shared between them.
Complete step-by-step answer:
The atomic number of Beryllium is four. So Beryllium needs four more electrons to fill its octet.
A) For this molecule, BeCl2 exists as a dimer. The structure for this compound is as follows.
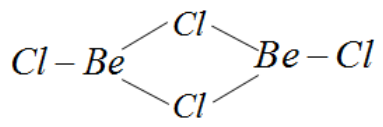
Beryllium needs four more electrons to attain octet or noble gas configuration but it is clear that Beryllium chloride as a dimer only has three bonds around Beryllium. Hence this compound does not complete octet configuration.
B) Similar to Beryllium chloride, BeH2 as a dimer does not attain octet configuration.
C) In solid phase, the compound Beryllium hydride forms a polymer. This polymer is formed by Hydrogen bridging. The structure for this compound or molecule can be given as follows.
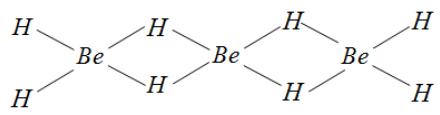
Since Beryllium contains only two electrons and all the bonds formed here are covalent bonds, it cannot attain octet.
D) This compound also exists as a polymer with Chloride atoms bridging. The structure for this compound is:
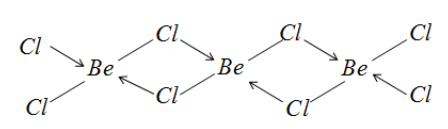
Due to the Chlorine atom bridging and the dative bonds, Beryllium acquires eight electrons around it and hence attains octet.
Therefore, the correct answer is option D.
Note: It is to be noted that since Beryllium contains only two valence electrons, it can never attain octet by ionic or covalent bonds i.e. sharing of electrons. Instead, it attains octet through dative bonds or coordinate covalent bonds.
