Question
Question: Which of the following molecules has a tetrahedral shape? (A)- \({\text{HgC}}{{\text{l}}_{\text{2}...
Which of the following molecules has a tetrahedral shape?
(A)- HgCl2
(B)- CO2
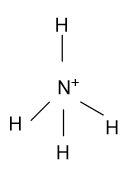
(C)- NH4 +
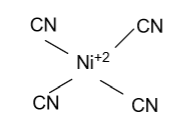
(D)- Ni(CN)42 -
Solution
Molecule whose shape is tetrahedral, is having four hybrid orbitals and the hybridization of central atom in that molecule is sp3 and bond angle between two bonds is around 1090.
Complete answer: In the above question following molecules are given and their hybridization is as follow:
-In HgCl2, mercury is the central atom whose atomic number is 80 & two electrons are present in the outermost shell which binds with the 2 chlorine atoms and form HgCl2 whose hybridization is sp & having linear shape.
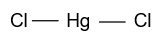
-In CO2, carbon is the central atom whose atomic number is 6 & four electrons are present in the outermost shell which binds with the 2 oxygen atoms through double bond and form CO2 whose hybridization is sp & having linear shape.
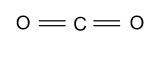
-In NH4 + , Nitrogen is the central atom whose atomic number is 7 & five electrons are present in the outermost shell but here nitrogen is present in +1 oxidation state it means nitrogen remove one electron from the outermost shell. Now the four electrons binds with the 4 hydrogen atoms and form NH4 + whose hybridization is sp3 & having tetrahedral shape.
-In Ni(CN)42 - , Nickel is the central atom whose atomic number is 28 & two electrons are present in the outermost shell but here nickel is present in +2 oxidation state it means Nickel removes two electrons from the outermost shell. In the given molecule CN is the strong ligand who tries to pair all unpaired electrons of the d-orbital & we get one empty d-orbital. Now the four empty orbitals binds with the 4 CN atoms and form Ni(CN)42 - whose hybridization is dsp2 & having square planar shape.
Hence, option (C) is correct.
Note: In this question some of you may give the wrong answer by considering the option (D) molecule by keeping the fact that it is also making four bonds so that it will also show tetrahedral structure. But, that is not true because in the transition metal molecules we have to give attention to the ligand present on that molecule.
