Question
Question: Which of the following molecules has a tetrahedral shape? A. \(HgC{l_2}\) B. \(C{O_2}\) C. \(...
Which of the following molecules has a tetrahedral shape?
A. HgCl2
B. CO2
C. NH4+
D. Ni(CN)42−
Solution
VSEPR helps in predicting the geometry of a compound taking in account the arrangement of electron pairs. VSEPR theory states that the electrons present around repel each other and it tends to take up an arrangement that will have minimum repulsion.The number of the valence shell present in the central metal atom is determined by drawing a Lewis structure of that atom.
Complete step by step answer:
NH4+ has sp3 hybridization, that is, it involves one s atomic orbital and three p atomic orbital. It has a bond angle of 109.50. N has the electronic configuration [He]2s22p3 and hydrogen has the electronic configuration 1s2. So, using this, we can now draw the tetrahedral structure of NH4+ in the following way.
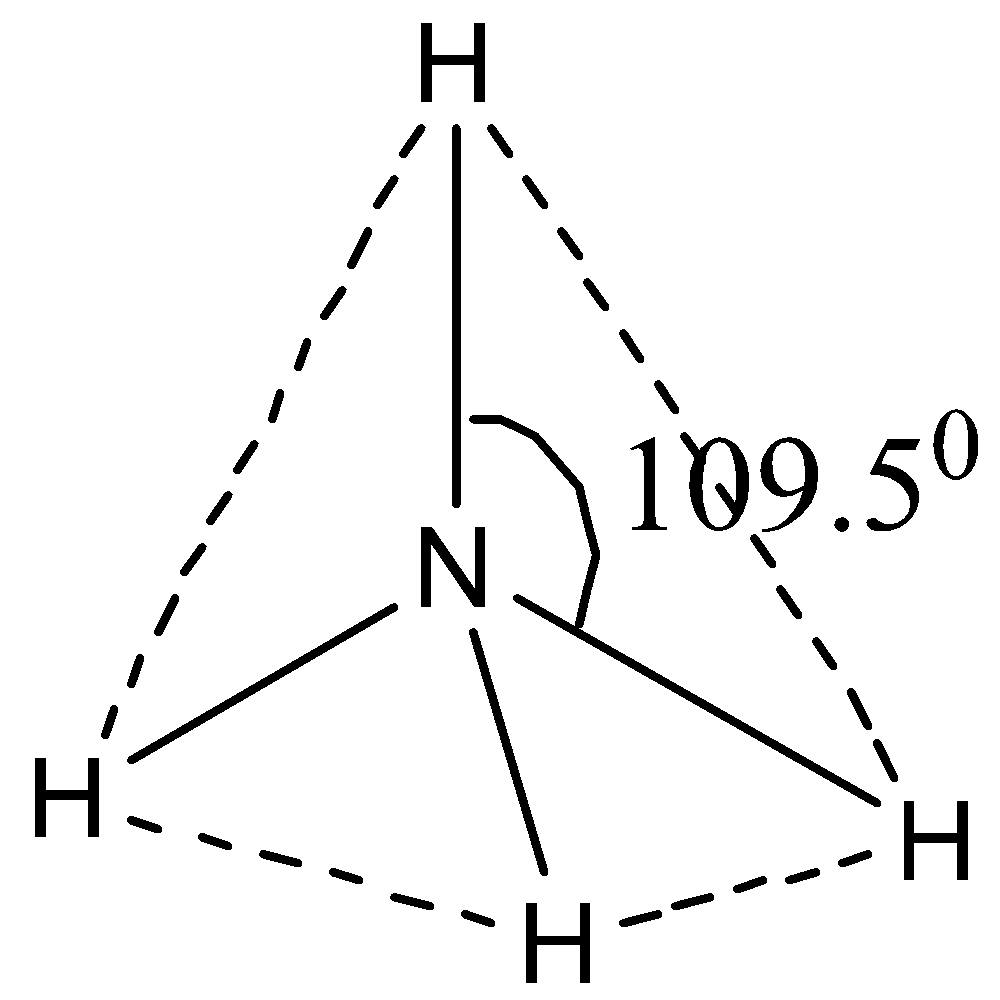
Therefore, NH4+ it has a tetrahedral shape.
HgCl2 and CO2 involves sp hybridisation. Therefore, both of them have a linear structure.
Ni(CN)42− has dsp2 hybridisation. It has a square planar geometry.
Hence C is the correct option.
Note:
The steric number of the central atom in the molecule is given as the number of atoms bonded to the central atom, which is called as the summation of the coordination number and the number of lone pairs of valence electrons on the central atom.
