Question
Question: Which of the following molecules does not exhibit dipole moment?...
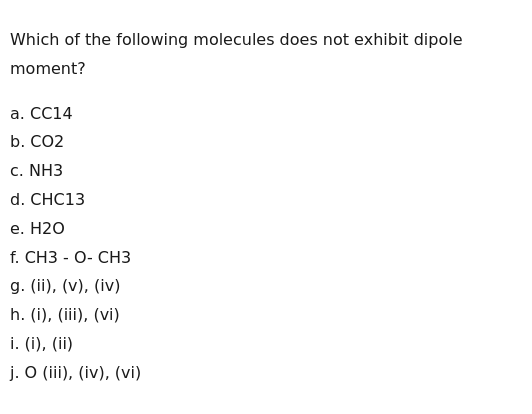
Which of the following molecules does not exhibit dipole moment?
A
CC14
B
CO2
C
NH3
D
CHC13
E
H2O
F
CH3 - O- CH3
G
(ii), (v), (iv)
H
(i), (iii), (vi)
I
(i), (ii)
J
O (iii), (iv), (vi)
Answer
(i), (ii)
Explanation
Solution
For a molecule to be nonpolar, its geometry must be symmetric so that individual bond dipoles cancel out.
- CCl₄: Tetrahedral symmetry; all C–Cl bond dipoles cancel, resulting in no net dipole moment.
- CO₂: Linear geometry with two identical C=O bonds in opposite directions; dipoles cancel.
The other molecules have asymmetry leading to net dipole moments.
