Question
Question: Which of the following molecule is linear: (A) \(S{{O}_{2}}\) (B) \(N{{O}_{2}}^{+}\) (C) \(N{{...
Which of the following molecule is linear:
(A) SO2
(B) NO2+
(C) NO2
(D) SCl2
Solution
The 3-D arrangement of atoms in a molecule is known as its molecular geometry. The molecular geometry includes the shape of the molecule as well as the bond length and bond angles of the molecule. All these factors help to determine the position of each atom in the geometry.
Complete step by step solution:
VSEPR theory helps us to predict the geometry of the molecule from the number of electron pairs and lone pairs around the central atom.
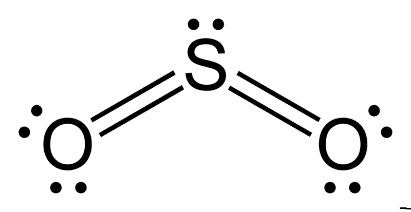
For SO2
S has 6 unpaired electrons and oxygen is a divalent atom.
Hybridization can be calculated by adding sigma bond and lone pairs.
S has 1 lone pair and form 2 sigma bond: hybridization = 1+2=3
And hybridization will be sp2 and shape is v shaped
For NO2+
N has 5 unpaired electrons, due to positive charge N will have 4 unpaired electrons and oxygen is a divalent atom.
Hybridization can be calculated by adding sigma bonds and lone pairs.
N has 0 lone pair and form 2 sigma bond: hybridization = 0+2=2
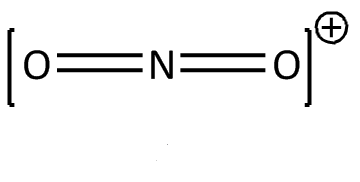
Hence the correct option is (B)
Note: Similarly for NO2 hybridization will be sp2 and shape is bent and for SCl2 hybridization will be sp3 and shape is bent .The geometry and shape of a molecule can be same or different as geometry of the molecule depends on the arrangement of lone pair and bond Pair while the shape of a molecule exclude the lone pair on the central atom.
