Question
Question: Which of the following methods is used to estimate the hardness of water? (A). Conductivity method...
Which of the following methods is used to estimate the hardness of water?
(A). Conductivity method
(B). EDTA method
(C). Titrimetric method
(D). Distillation method
Solution
Hard water has a high amount of dissolved minerals like calcium and magnesium. Hard water is formed when water percolates through deposits of limestone, chalk or gypsum which are largely made up of calcium and magnesium salts of carbonates, bicarbonates and sulphates. Hardness is measured in milligrams of calcium carbonate present per litre.
Complete step by step answer:
The hardness of water is caused due to the presence of dissolved magnesium and calcium salts. However, due to the presence of dissolved calcium or magnesium sulphates, permanent hardness of water is caused because these salts cannot be removed by boiling. On the other hand, the dissolved magnesium and calcium bicarbonates cause temporary hardness in water as they can be removed by boiling the water.
The hard water buildup in pipes causes less water flow due to dropped pressure. The hard water also builds up on geysers and other elements and damages them as the heating efficiency of these elements get reduced and this will increase the consumption of electricity. Moreover, the hard water reduces the lathering properties of soaps and detergents, causing the more use of these products. There are many more disadvantages of using hard water.
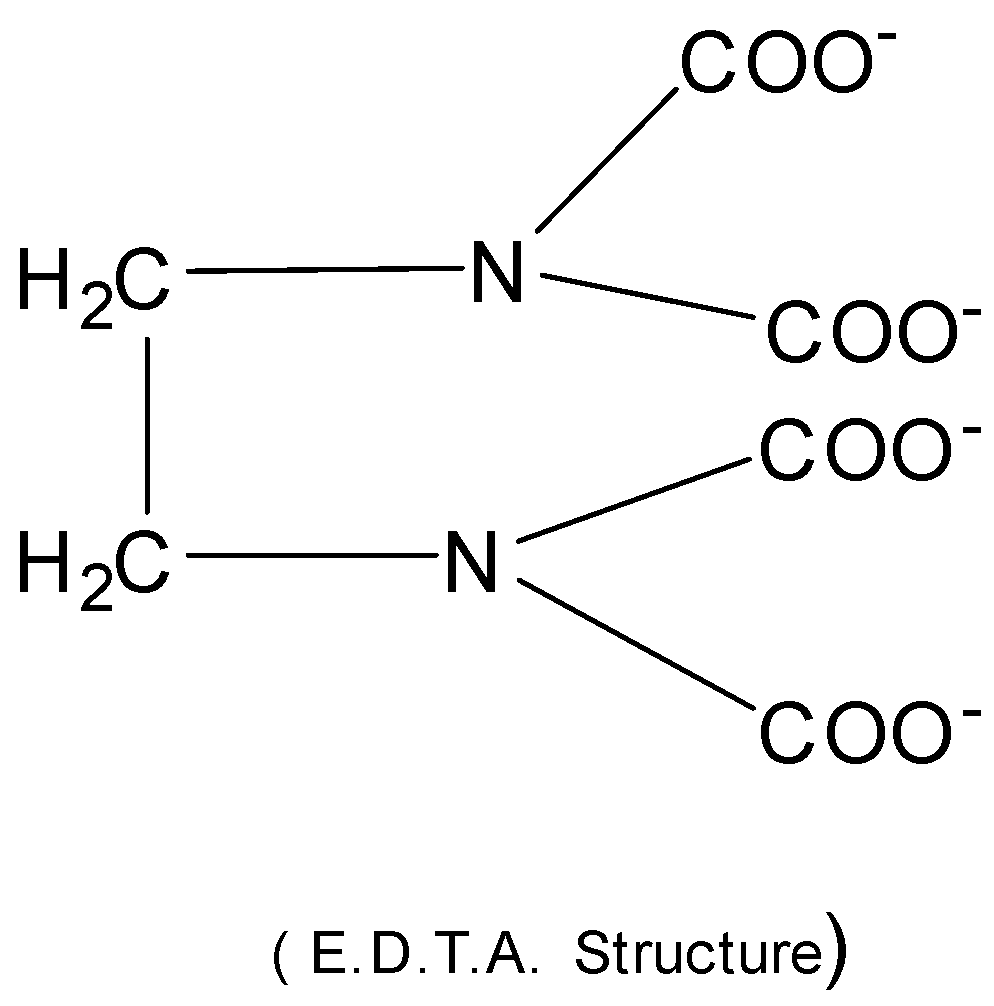
So, it is important to estimate the hardness of water so as to minimize the effect caused by hard water. The hardness of water can be determined by the complexometric titration using Ethylene diamine tetra acetic acid ( EDTA ) . EDTA in the form of its di− sodium salt creates a complex with Ca2+ and Mg2+ ions of water. Then Ca2+ and Mg2+ ions form stable metal complexes with EDTA and the color changes from wine red to blue at the end point.
So, the correct answer is Option B.
Note:
EDTA is a multidentate ligand
It’s denticity (multiplicity) is 6.
EDTA coordinates through 40 atoms and 2N atoms.