Question
Chemistry Question on Solutions
Which of the following liquid pairs shows a positive deviation from Raoult's law?
A
Water - hydrochloric acid
B
Acetone - chloroform
C
Water - nitric acid
D
Benzene - methanol
Answer
Benzene - methanol
Explanation
Solution
A positive deviation from Raoult's law occurs when the vapor pressure of a solution is higher than what would be expected based on the ideal behavior predicted by Raoult's law. The vapor pressure of each component is directly proportional to its mole fraction in the solution.
Benzene in methanol breaks the H - bonding of the alcohol making its boiling point decrease & there by its vapour pressure increases leading two +ve deviation.
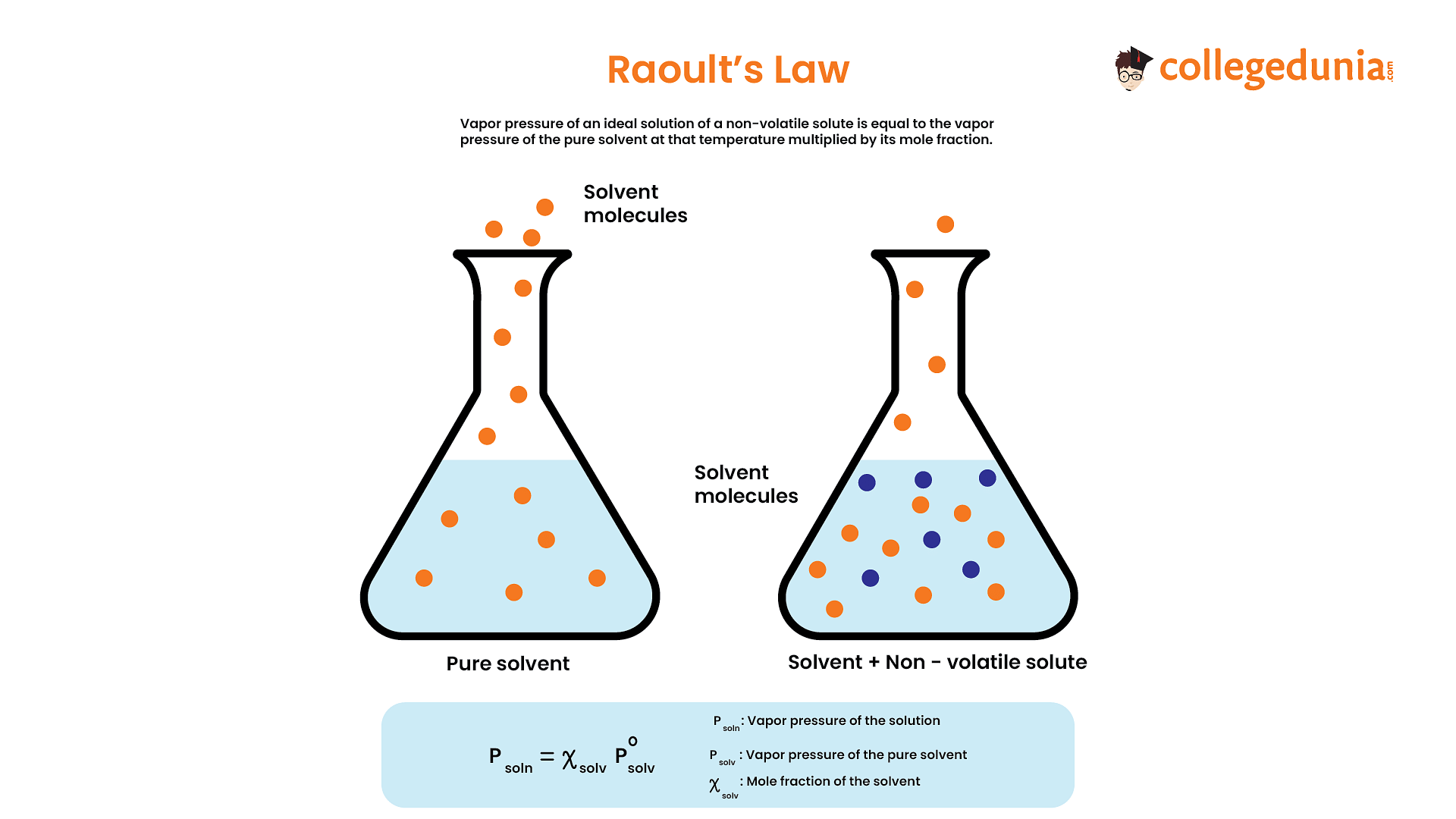
Causes of positive deviation from Raoult’s law
- Intermolecular forces
- Molecular size and shape
- Hydrogen bonding
Read more from chapter:**Solutions **
