Question
Question: Which of the following Lewis dot structures of \(C{{O}_{2}}\) is correct? A.
B.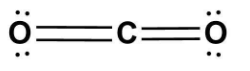
C.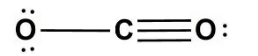
D. None of these.
Solution
The Lewis structure of any compound is comprised of the atoms involved in the forming of that molecule; the number of valence electrons present (either in the form of bond pair or lone pair) in each atom are described in the Lewis structure.
Complete answer: Let us try and draw the Lewis structure of CO2;
The CO2 molecule consists of Carbon and Oxygen as the atoms involved in the process. We firstly need to find the number of electrons which they have in their valence shells.
Carbon has 4 valence electrons which can be involved in the bond formation. Whereas, Oxygen has 6 electrons which can either be involved in bond formation or can be paired as lone pairs.
So, to complete the octet, each of the Oxygen will bind to Carbon atom forming two double bonds satisfying both the sides of the molecule. The remaining electrons will form the lone pairs on the oxygen.
Thus, the Lewis structure of CO2 will look like;
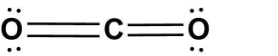
which will resemble option (B).
Note: There are specific steps to draw a Lewis structure for a molecule as described above giving a proper and correct answer. This can be also done by analysing the options given in the question;
In option (A)- Oxygen atom cannot have 3 lone pairs of electrons as it has a bond with carbon.
In option (C)- Neither of the oxygen has satisfied the valency it has.
Thus, they can never be the answers.
