Question
Question: Which of the following kinds of isomerism can nitroethane exhibit? A.Metamerism B.Optical isomer...
Which of the following kinds of isomerism can nitroethane exhibit?
A.Metamerism
B.Optical isomerism
C.Tautomerism
D.Position isomerism
Solution
first we have to make the structure of nitroethane and then we should check which of the following properties of isomerism will exhibit.
Complete step by step answer:
The structure of nitroethane is the following
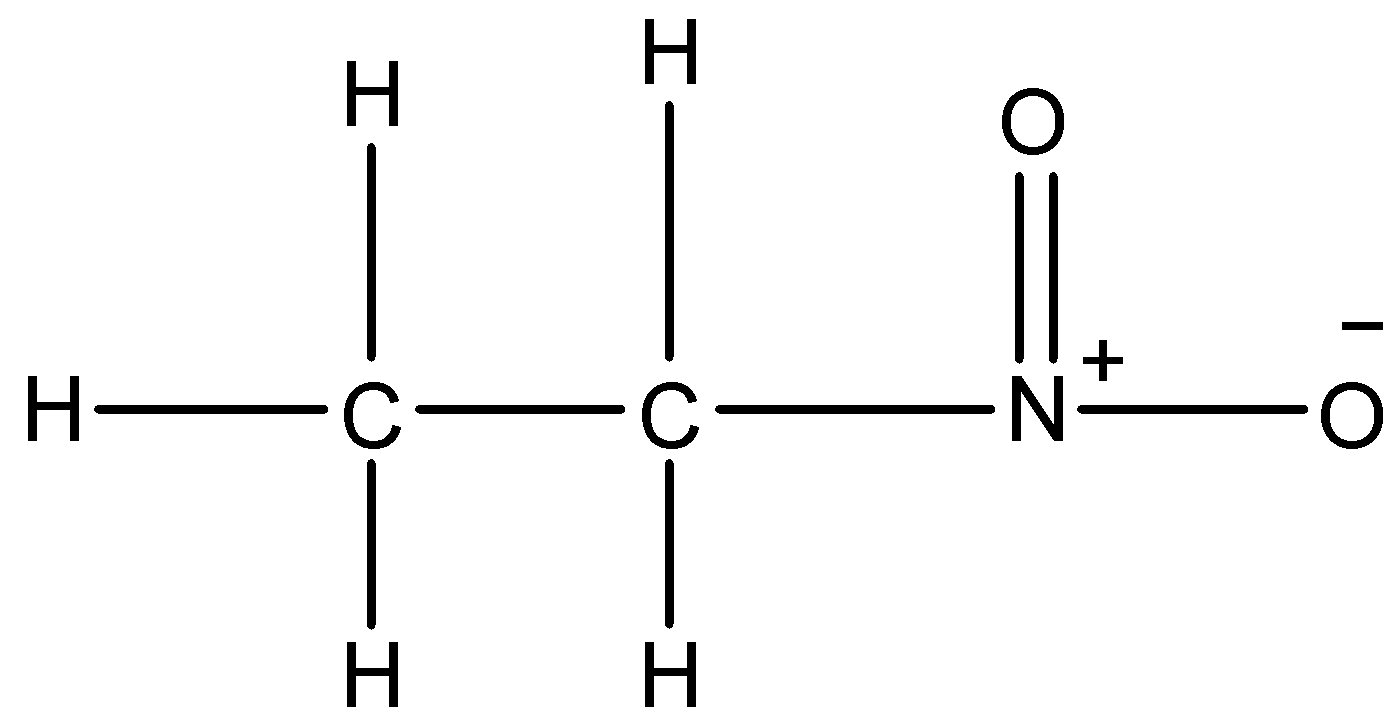
The following structure will not show metamerism because in metamerism we need to have carbon chains on both the side of the functional group. But in this case we have a carbon chain only at one side, so metamerism is not exhibited by nitroethane.
The nitroethane does not exhibit optical isomerism because for optical isomerism the condition is that the compound should contain at least one chiral carbon atom. A chiral carbon atom is one which has four different groups attached to it and is asymmetrical. Here we don’t have any chiral carbon atoms.
The compound exhibits tautomerism that is option c because it has an acidic hydrogen with it. The acidic hydrogen is attached to a second carbon atom. The compound formed after tautomerism is the following
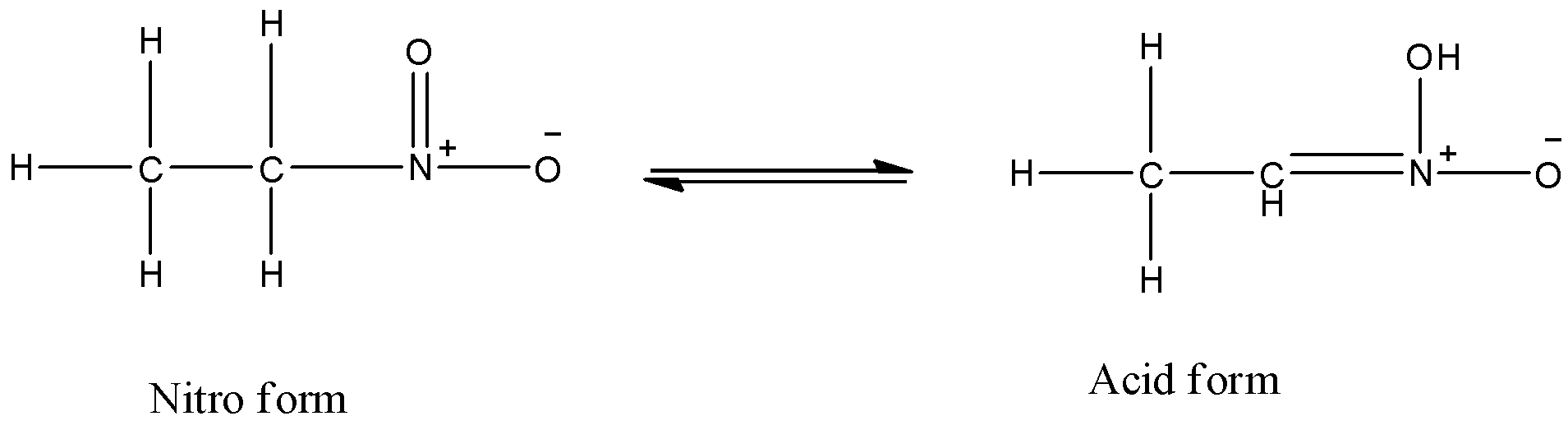
The nitroethane will not show position isomer because if we attach the nitro group to the other carbon atom then also the nitro group will occupy first position.
Hence, option C is the correct answer.
Note: Metamerism is a structural isomerism in which different alkyl groups are attached to the same functional group. Optical isomerism is the isomerism in which two compounds have the same number and same kind of atoms and the connectivity of bond is also same but the difference is in its spatial arrangement.
