Question
Question: Which of the following isomer is meso: A. 
B.
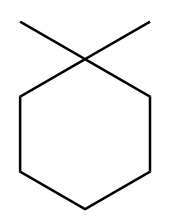
C.
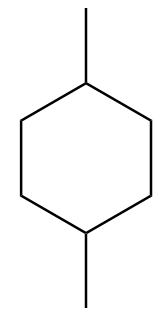
D. None of these
Solution
An object is said to be a chiral object if it has a non-superimposable mirror image and if the mirror image of the object is superimposable to its original structure, then it is said to be achiral. Let’s try to visualize chiral and achiral objects through an example which is given as follows:
Chiral object:
Achiral object:
Complete answer:
In organic chemistry, the compounds which are non-optically active members of the chiral compounds or optically inactive members of the set of stereoisomers are known as meso compounds. For a compound to be a meso isomer, following two points must strictly be followed:
1.The compound must have at least two chiral centres within its molecular structure.
2.The mirror image of the molecule must be non-superimposable to the original structure of the molecule i.e., it must be a chiral molecule.
3.It must possess a plane of symmetry or centre of symmetry within its structure i.e., there must be a possibility in which a plane can divide the molecule in two equal halves.
Now, among given options let us check the possibility of the molecule to be meso for each isomer separately.
Isomer-A:
The mirror image for an isomer-A is formed as follows:
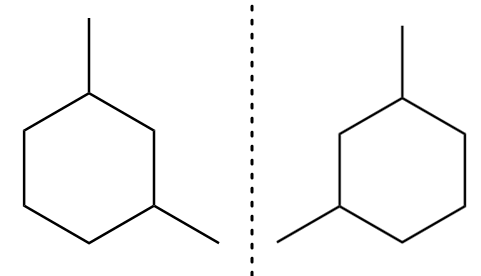
As the mirror image for the isomer-A is non-superimposable to its original structure. Therefore, the molecule is chiral and there exist a plane of symmetry within its molecular structure as follows:
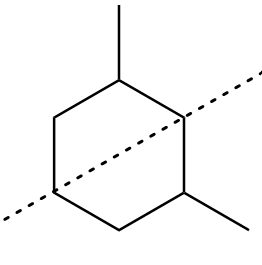
Hence, the given isomer is a meso compound.
Isomer-B:
The mirror image for an isomer-B is formed as follows:
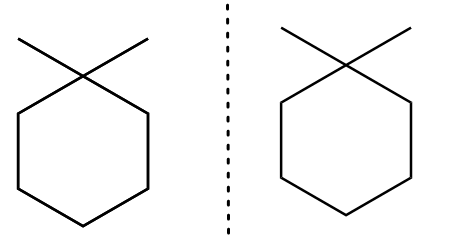
As the mirror image for the isomer-B is superimposable to its original structure. Therefore, the molecule is achiral and therefore, it is not a meso compound.
Isomer-C:
The mirror image for an isomer-C is formed as follows:
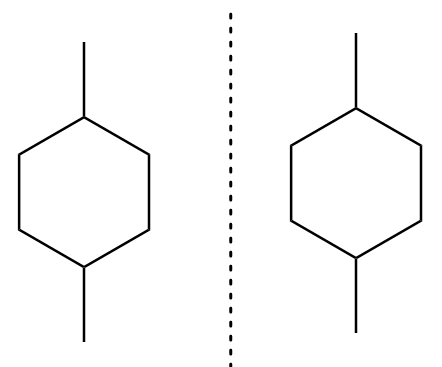
As the mirror image for the isomer-C is superimposable to its original structure. Therefore, the molecule is achiral and therefore, it is not a meso compound.
Hence, option (A) is the correct answer.
Note:
Remember that the isomer-A will possess plane of symmetry only when it is present in its cis-form i.e., the substituent groups attached to the ring must present in the same plane to be a meso compound. If the substituent groups are bonded via different planes, then the molecule is optically active and will not possess any plane of symmetry.
