Question
Question: Which of the following is/are resolvable ? ...
Which of the following is/are resolvable ?
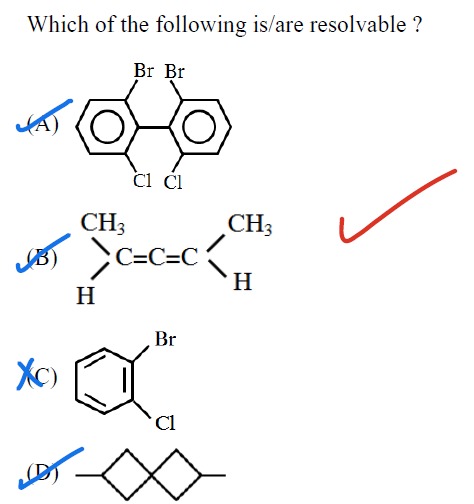
Substituted biphenyl with Br and Cl substituents at ortho positions
Allene derivative: CH3−CH=C=C−H(CH3)
1-bromo-2-chlorobenzene
Spiro compound with two spiro junctions and terminal methyl groups
A, B, D
Solution
The question asks which of the given molecules are resolvable. Resolvable molecules are chiral molecules that can be separated into their enantiomers. Chirality is the property of a molecule that is non-superimposable on its mirror image. A molecule is chiral if it lacks symmetry elements such as a plane of symmetry (σ) or a center of symmetry (i).
-
(A) Substituted biphenyl: The substituents are at the ortho positions (2, 2', 6, 6'). The rotation around the central C-C bond is restricted, leading to atropisomerism. This molecule is chiral and hence resolvable.
-
(B) Allene derivative: CH3−CH=C=C−H(CH3). For an allene to be chiral, the two groups on one terminal carbon must be different, and the two groups on the other terminal carbon must be different. Therefore, this allene is chiral and resolvable.
-
(C) 1-bromo-2-chlorobenzene: Benzene and its derivatives are planar molecules. The plane of the benzene ring is a plane of symmetry for this molecule. Therefore, this molecule is achiral and not resolvable.
-
(D) Spiro compound: The presence of terminal methyl groups can lead to chirality if there is no plane of symmetry or center of symmetry. This molecule is likely chiral and resolvable.
Based on the analysis, molecules A, B, and D are resolvable, while molecule C is not.
