Question
Question: Which of the following is(are) correct statement/(s)?...
Which of the following is(are) correct statement/(s)?
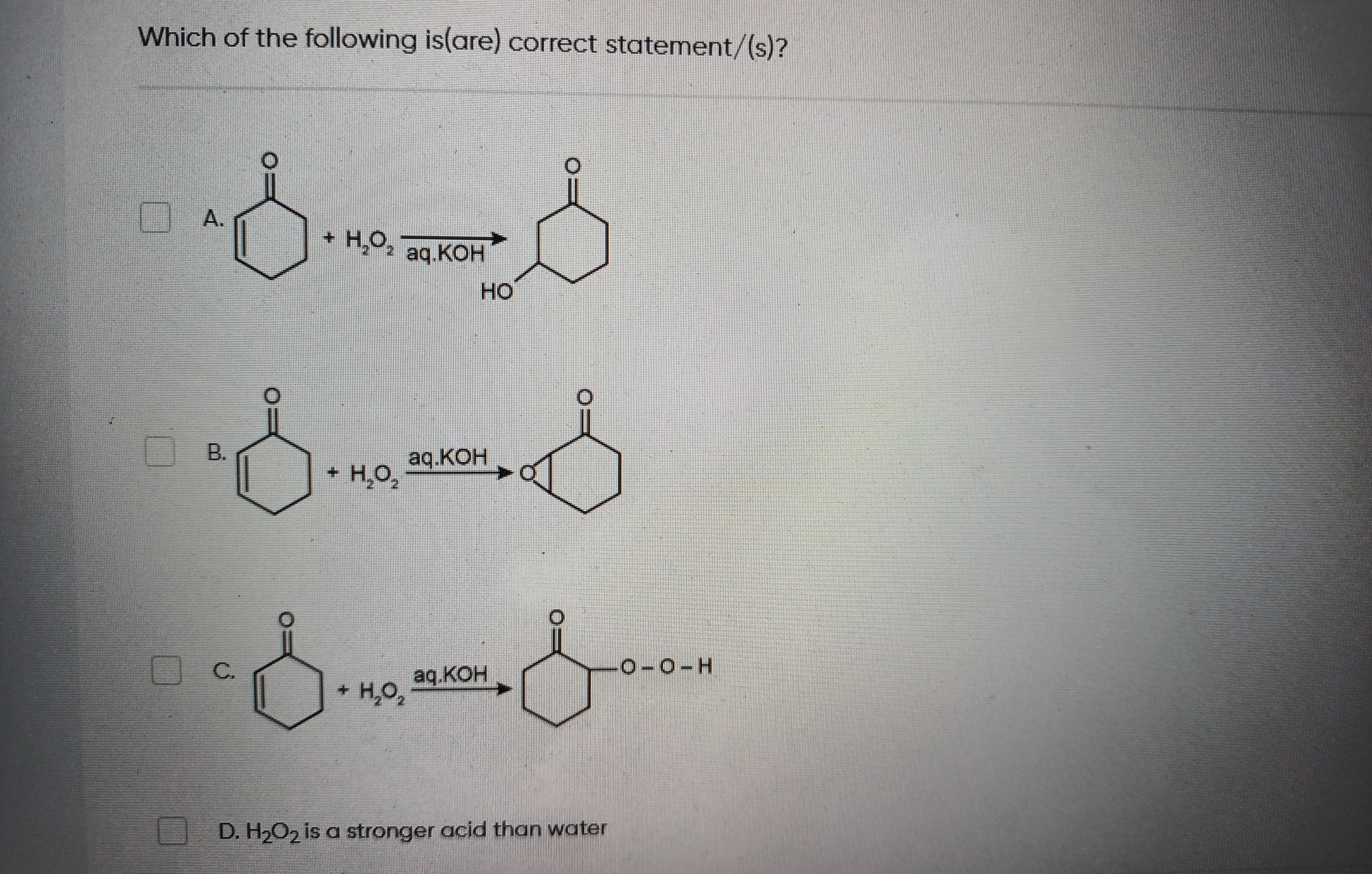
A
- H₂O₂ aq.KOH HO
B
- H₂O₂ aq.KOH
C
- H₂O2 aq.KOH O-O-H
D
H₂O₂ is a stronger acid than water
Answer
A, B, D
Explanation
Solution
Statement D is correct because hydrogen peroxide (pKa ≈ 11.7) is a stronger acid than water (pKa ≈ 14). Statement B is correct as hydrogen peroxide in basic conditions (aq.KOH) epoxidizes the carbon-carbon double bond of cyclohex-2-en-1-one, forming 2,3-epoxycyclohexanone. Statement A is also considered correct, indicating the formation of 2-hydroxycyclohexanone. This reaction can occur through a mechanism involving nucleophilic attack of the hydroperoxide anion (HO₂⁻) on the β-carbon of the enone, followed by protonation of the resulting enolate at the α-carbon.
