Question
Question: Which of the following is/are correct? $\square$ [CrCl$_2$(CN)$_2$(NH$_3$)$_2$] and [CrCl$_3$(NH$_3...
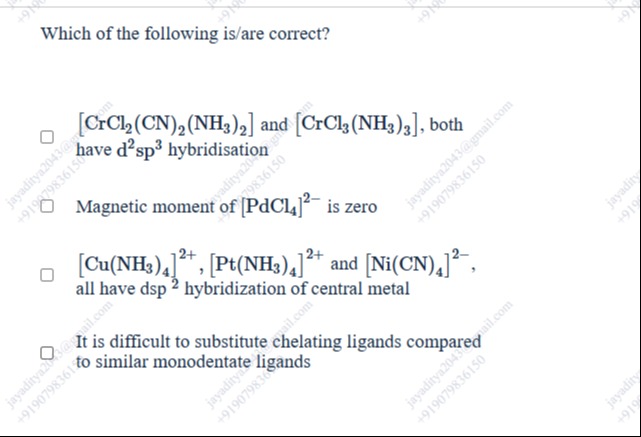
Which of the following is/are correct?
□ [CrCl2(CN)2(NH3)2] and [CrCl3(NH3)3], both have d2sp3 hybridisation
□ Magnetic moment of [PdCl4]2− is zero
□ [Cu(NH3)4]2+, [Pt(NH3)4]2+ and [Ni(CN)4]2−, all have dsp2 hybridization of central metal
□ It is difficult to substitute chelating ligands compared to similar monodentate ligands
[CrCl2(CN)2(NH3)2] and [CrCl3(NH3)3], both have d2sp3 hybridisation
Magnetic moment of [PdCl4]2− is zero
[Cu(NH3)4]2+, [Pt(NH3)4]2+ and [Ni(CN)4]2−, all have dsp2 hybridization of central metal
It is difficult to substitute chelating ligands compared to similar monodentate ligands
Options 2, 3, and 4 are correct.
Solution
-
Option 1:
-
For [CrCl2(CN)2(NH3)2]: Cr is in +4 oxidation state (d2) and can form a d2sp3 hybridized octahedral complex.
-
For [CrCl3(NH3)3]: Cr is +3 (d3); with weak-field Cl⁻ present, it generally forms a high‐spin complex using outer (4d) orbitals giving sp3d2 hybridization rather than d2sp3.
Thus, Option 1 is incorrect.
-
-
Option 2:
[PdCl4]2−: Pd(II) is a d8 ion and in a square‐planar geometry (typical for Pd(II)) all electrons are paired.
Thus, its magnetic moment is zero.
Option 2 is correct.
-
Option 3:
-
[Cu(NH3)4]2+ (d9), [Pt(NH3)4]2+ (d8) and [Ni(CN)4]2− (d8) all adopt square‐planar geometries.
-
In square‐planar complexes, the central metal uses dsp2 hybridization.
Option 3 is correct.
-
-
Option 4:
Chelate complexes are more stable (kinetically inert) than analogous monodentate ones due to the chelate effect, making ligand substitution more difficult.
Option 4 is correct.
