Question
Question: Which of the following is zero overlapping which leads to non-bonding? A.
B.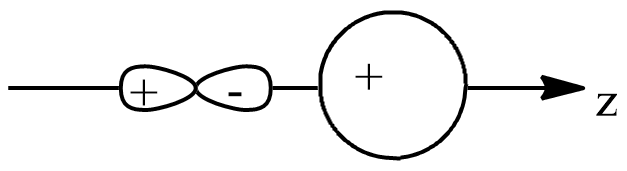
C.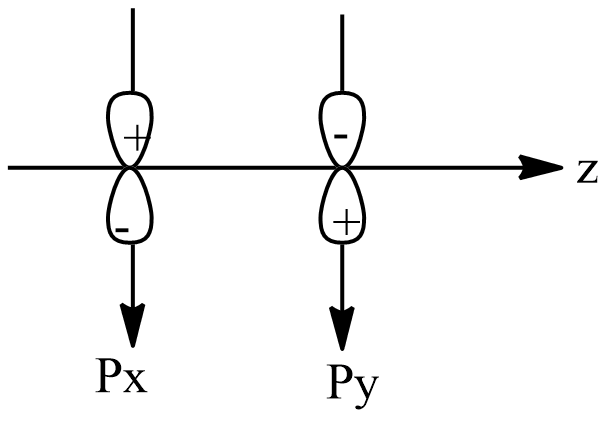
D.All
Solution
Zero overlapping occurs when both the orbitals do not overlap with each other. Zero overlapping occurs in two conditions, one of them is that both the orbitals are not in the same plane and other is that the orbitals should not be symmetrical.
Complete answer:
Zero overlapping is something in which there is no overlapping between two orbitals.
Now, zero overlapping occurs when one of two conditions are satisfied.
The first condition is that the two orbitals should not be symmetrical and the second condition is that both the orbitals should be in different planes.
Now, option A shows that both the orbitals are in different planes, x and y. Thus, both the orbitals are perpendicular and cannot overlap. As one of the conditions is satisfied, zero overlapping will occur, which will lead to non-bonding.
If we see other options, in option B, both the orbitals are in the same plane, thus one condition is failed. Also, the positive orbital overlaps with the negative orbital, which makes it negative overlapping.
In option C, they do not seem to be perpendicular, thus they are in the same plane and negative orbital overlaps with positive orbital, which makes it negative overlapping.
As option B and C are discarded, option D is automatically discarded, thus the correct option is option A.
Therefore, the correct answer is option A.
Note:
If any condition from the two conditions for zero overlapping is satisfied, then it will occur in zero overlapping, thus it is necessary to check both the conditions for every option. Here, option B and C had no conditions satisfied.
