Question
Question: Which of the following is used as Hinsberg’s reagent? (A) \({{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\) (B...
Which of the following is used as Hinsberg’s reagent?
(A) C6H5SO2Cl
(B) C6H5SO3H
(C) C6H5NHCH3
(D) C6H5COCH3
Solution
Hint: Knowing the scientific name of Hinsberg’s reagent will help. It is an aromatic compound with a sulfonyl group.
Complete step-by-step answer:
Hinsberg’s reagent is used to distinguish between primary, secondary and tertiary amines. Its chemical formula is benzene sulphonyl chloride (C6H5SO2Cl). Its structure is as below:
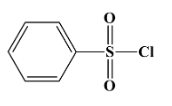
The correct answer for the above question is option (A)C6H5SO2Cl.
Additional information:
Let’s know more about the reactions using Hinsberg’s reagent that distinguish the amines of different degrees.
- When a primary amine says ethyl amine reacts with this reagent, we get N−methylbenzenesulfonamide. This product is soluble in alkali due to the highly acidic hydrogen atom which is shown in red here:
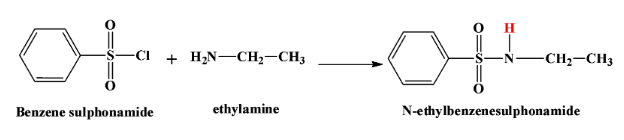
- When a secondary amine reacts with this reagent, there is no hydrogen atom left in the product which is N,N−diethylbenzenesulphonamide. Therefore the product is not soluble in alkali. The reaction is as below:
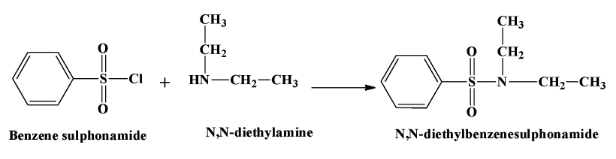
- Tertiary amines do not react with benzene sulphonyl chloride.
In this way we can separate or distinguish primary, secondary and tertiary amines.
Note: In case of primary amines, when it is said that a product will dissolve in any solution, it just means that a reaction will take place and no precipitate will be left behind. If this is an acid-base reaction, as is the above case, we can get back the reactants if we want to. In this way we can separate our required compound from a mixture.
In case of secondary amines, the alkali cannot react with the product formed. This means there will be no reaction taking place when the alkali is added.
