Question
Question: Which of the following is used as a fire extinguisher? A.Sodium carbonate B.Sodium bicarbonate ...
Which of the following is used as a fire extinguisher?
A.Sodium carbonate
B.Sodium bicarbonate
C.Sodium chloride
D.Ammonium chloride
Solution
Fire extinguisher is a device used by the fire brigade person to control fire. They generally cut off the supply of oxygen to control fire. To solve this question, we need to know different types of fire extinguishers and the chemicals used in them.
Completes step by step answer:
Basically, fire is one of the most useful phenomena we exploit in our day to day life to accomplish various tasks. Some of the examples are, for cooking, for illumination etc. Moreover, the fire which is boon for us can turn into a bane too if it is not controlled, for example forest fires. So, to control such fires, we generally call the fire brigade and they generally use fire extinguishers to extinguish the fire depending on its magnitude.
Now, the fire extinguisher is a device used by the fire brigade person to control fire. Basically, according to the concept of fire triangle, for the generation of fire, we need three things to be present simultaneously i.e. fuel, a heat source and air. If we remove any of these resources, then the fire is controlled. Further, the fire extinguishers generally cut off the supply of oxygen to control fire. The fire triangle is as shown:
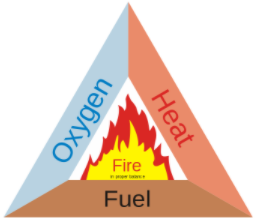
Now, among the following options, sodium bicarbonate also known as sodium hydrogen carbonate is used as the main constituent of dry-chemical fire extinguishers. When the fire extinguisher is released by pressing the knob on it, then the sulfuric acid mixed with sodium bicarbonate solution produces a lot of carbon dioxide gas. This gas is heavier than air and offsets the supply of oxygen from air and hence removes the fire. The equation is as shown:
2NaHCO3+H2SO4→Na2SO4+2H2O+2CO2
Hence, option B is correct.
Note: You may have seen that the fire brigade person sprays water at the place where the fire takes place. This is because water starts to cool down the temperature of the fire. As the temperature starts to fall down, due to continuous spraying of water, it finally comes down below the ignition temperature of the fuel. So, the fuel cannot be ignited anymore and thus prevents the fire from spreading. Also, the water vapor that is generated, blocks the supply of oxygen to the fuel surface and thereby decreases the supply of oxygen.
