Question
Question: Which of the following is true for any diprotic acid \({{H}_{2}}X\)? (A) \({{K}_{{{a}_{2}}}}>{{K}_...
Which of the following is true for any diprotic acid H2X?
(A) Ka2>Ka1
(B) Ka1>Ka2
(C)Ka2=Ka11
(D) Ka2=Ka1
Solution
Ka1 and Ka2 are acid constants for polyprotic acids, which may consist of diprotic acids, triprotic acids, etc.
Complete step by step answer:
Let us first understand about the diprotic acids;
Diprotic acid- A diprotic acid is an acid that can donate two hydrogen ions (H+) or protons per molecule in an aqueous solution. Another name for diprotic acid is dibasic acid. A diprotic acid is a type of polyprotic acid, which is an acid able to donate more than one proton per molecule.
Several important acids can be classified as polyprotic acids, which can lose more than one H+ ion when they act as Bronsted acids. Diprotic acids, such as sulphuric acid (H2SO4), carbonic acid (H2CO3), hydrogen sulphide (H2S), chromium acid (H2CrO4), and oxalic acid (H2C2O4) have two acidic atoms. Triprotic acids, such as phosphoric acid (H3PO4) and citric acid (C6H8O7), have three.
Diprotic acids-
A diprotic acid (here symbolised by H2X) can undergo one or two dissociations depending on pH. Dissociation does not happen all at once; each dissociation step has its own Ka value, designated Ka1and Ka2.
H2X(aq)⇄H+(aq)+HX−(aq) Ka1
HX−(aq)⇄H+(aq)+X2−(aq) Ka2
The first dissociation constant is necessarily greater than the second (i.e. Ka1>Ka2); this is because the first proton to dissociate is always the most strongly acidic, followed in order by the next most strongly acidic proton.
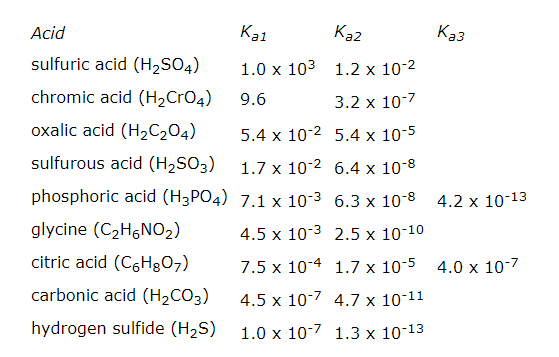
Let us see for the sulphuric acid, who can donate two protons in the solution;
H2SO4(aq)→H+(aq)+HSO4−(aq) Ka1 = largeHSO4−(aq)⇄H+(aq)+SO4−(aq) Ka2 = small
This first dissociation step of sulphuric acid will occur completely, which is why sulphuric acid is considered as strong acid; the second dissociation step is only weakly dissociating, however.
So, generally for any polyprotic acid, the first acid constant will always be greater than rest as after losing one proton it becomes difficult to lose another electron and act as acid.
So, the correct answer is “Option B”.
Note: Do note that the trend follows for any polyprotic acid. It’s not limited to only diprotic acids.
The dissociation constant trend is the same for all diprotic acids and this follows for other polyprotic acids too.
