Question
Question: Which of the following is the wrong statement? A) In oxymercuration and demercuration reaction, al...
Which of the following is the wrong statement?
A) In oxymercuration and demercuration reaction, alcohol is formed by Markovnikov addition.
B) In hydroboration oxidation reaction, alcohol is formed by anti-Markovnikov addition.
C) Regiospecific of HBO reaction is syn.
D) Regiospecificity of oxymercuration and demercuration reaction is anti-rearrangement occurs.
Solution
To solve the above question, through knowledge of the reaction of unsaturated hydrocarbons is required, and the knowledge of Markovnikov’s rule. It states that the OH groups attached to the most substituted carbon atom and hydrogen is attached to the least substituted carbon atom.
Complete step by step solution:
The oxymercuration and demercuration reaction can be used to turn an alkene into alcohol in the presence of mercury (II) acetate in tetrahydrofuran or (THF) followed by reduction with sodium borohydride. The reaction is not stereoselective and not subject to rearrangements as the reaction intermediate is not a carbocation but a mercurinium ion instead.
The mechanism follows Markovnikov’s rule of regioselectivity with the OH groups attached to the most substituted carbon atom and hydrogen is attached to the least substituted carbon atom. This is also a syn reaction where the mercurinium ion and the hydroxyl group lie on the same side of the molecule.
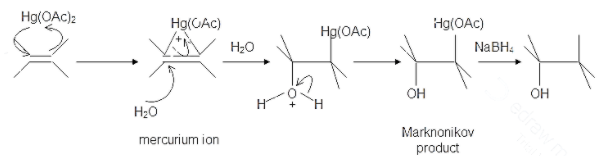
Hence, among all the options, option D is incorrect as in the regiospecificity of oxymercuration and demercuration reaction anti-rearrangement does not occur.
Note:
The Markovnikov's Rule state that in the addition of a protic solvent or an acid like hydrogen chloride to an unsaturated hydrocarbon like alkene or alkyne, the hydrogen atom of the solvent bonds to that carbon atom which has the higher number of hydrogen atoms.
Syn addition is the addition of two substituents on the same side of the double or triple bond whereas anti-addition refers to the addition of the substituents on different sides of the molecule.
So stereochemically, oxy-mercuration and demercuration is an anti- addition reaction where the nucleophile attacks the more substituted carbon atom as it retains more positive character than the less substituted carbon atom.
