Question
Question: Which of the following is the weakest bronsted base?...
Which of the following is the weakest bronsted base?
A

B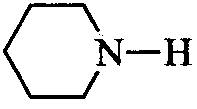
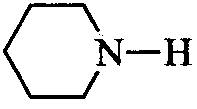
C

D
CH3NH2
Answer

Explanation
Solution
:  is the weakest Brönsted base due to delocalization of lone pair of electrons of N-atom into the benzene ring.
is the weakest Brönsted base due to delocalization of lone pair of electrons of N-atom into the benzene ring.
